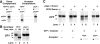A cell-free assay allows reconstitution of Vps33p-dependent transport to the yeast vacuole/lysosome - PubMed (original) (raw)
A cell-free assay allows reconstitution of Vps33p-dependent transport to the yeast vacuole/lysosome
T Vida et al. J Cell Biol. 1999.
Abstract
We report a cell-free system that measures transport-coupled maturation of carboxypeptidase Y (CPY). Yeast spheroplasts are lysed by extrusion through polycarbonate filters. After differential centrifugation, a 125,000-g pellet is enriched for radiolabeled proCPY and is used as "donor" membranes. A 15,000-g pellet, harvested from nonradiolabeled cells and enriched for vacuoles, is used as "acceptor" membranes. When these membranes are incubated together with ATP and cytosolic extracts, approximately 50% of the radiolabeled proCPY is processed to mature CPY. Maturation was inhibited by dilution of donor and acceptor membranes during incubation, showed a 15-min lag period, and was temperature sensitive. Efficient proCPY maturation was possible when donor membranes were from a yeast strain deleted for the PEP4 gene (which encodes the principal CPY processing enzyme, proteinase A) and acceptor membranes from a PEP4 yeast strain, indicating intercompartmental transfer. Cytosol made from a yeast strain deleted for the VPS33 gene was less efficient at driving transport. Moreover, antibodies against Vps33p (a Sec1 homologue) and Vam3p (a Q-SNARE) inhibited transport >90%. Cytosolic extracts from yeast cells overexpressing Vps33p restored transport to antibody-inhibited assays. This cell-free system has allowed the demonstration of reconstituted intercompartmental transport coupled to the function of a VPS gene product.
Figures
Figure 1
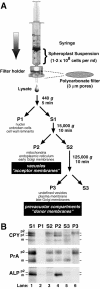
Lysis of yeast spheroplasts via extrusion through a polycarbonate filter. (A) Schematic diagram for polycarbonate filter lysis and subsequent differential centrifugation. After pushing a yeast spheroplast suspension in a syringe through a polycarbonate filter with 3-μm pores, the crude cell lysate was subjected to differential centrifugation using the indicated g forces and times. The P1, P2, and P3 pellets were enriched in the indicated organelles. (B) Fractionation of vacuolar marker proteins. Wild-type yeast spheroplasts (SEY6210) were radiolabeled with Tran35S-label for 5 min and chased with methionine and cysteine for 2 min at 30°C. The cells were subjected to lysis through a polycarbonate filter with subsequent differential centrifugation. Each supernatant and pellet from the 440 (S1 and P1), 15,000 (S2 and P2), and 125,000 g (S3 and P3) centrifugation steps was sequentially immunoprecipitated with antisera against carboxypeptidase Y (CPY), proteinase A (PrA), and alkaline phosphatase (ALP). Each immunoprecipitate was subjected to SDS-PAGE and autoradiography. The mid and late Golgi complex–modified precursor zymogen is designated p2, the endoplasmic reticulum and early Golgi complex precursor is designated p1 (CPY only), and the mature hydrolase is designated m for each protein.
Figure 2
Light microscopy of cell-free membrane pellets after polycarbonate filter lysis. Wild-type yeast cells (as in Fig. 1) were first stained with FM4-64 (15-min pulse, 45-min chase) followed with dichlorocarboxyfluorescein diacetate (15 min at pH 4.0) to mark the vacuole membrane and lumen, respectively. The double-stained cells were then enzymatically converted to spheroplasts at 30 or 15°C (as indicated). The spheroplasts were extruded through a polycarbonate filter with 3-μm pores (as described in Fig. 1 A). The lysate, P1, P2, and P3 pellets (as indicated) were then examined under a light microscope with differential interference contrast (DIC), phase contrast, and epifluorescence optics using a FITC and Texas red filter set (as indicated). The fluorescence images were digitally overlaid for a composite. The cells at 15°C (inset) were stained with just FM4-64 for a 30-min pulse. Bar, 5 μm.
Figure 3
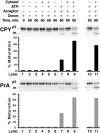
Vacuolar precursor proteins undergo maturation after incubating donor and acceptor membranes in a cell-free system. Wild-type yeast spheroplasts were radiolabeled (as in Fig. 1 B). The cells were subjected to lysis through a polycarbonate filter with subsequent differential centrifugation to generate a 125,000 g, P3 donor membrane pellet. The same yeast strain was used to make a 15,000 g, P2 acceptor membrane pellet from nonradiolabeled spheroplasts. The radiolabeled donor membranes (from ∼5 × 107 spheroplasts per reaction) were incubated at 25°C for 60 min with various combinations of buffer, ATP (plus a regeneration system), cytosol (5 mg/ml), and acceptor membranes (∼100 μg) in a total volume of 50 μl, as indicated. All reactions were sequentially immunoprecipitated for CPY and PrA, subjected to SDS-PAGE, and autoradiography. For the reactions in lanes 10 and 11, both donor and acceptor membranes were washed once with lysis buffer and reharvested before incubation with ATP and cytosol.
Figure 4
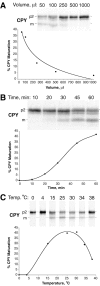
Characteristics of the cell-free system. Radiolabeled donor membranes and nonradiolabeled acceptor membranes were prepared and incubated (as described in Fig. 3) with modifications in reaction volume, time, or temperature. (A) Dilution sensitivity. All reactions were performed with a constant amount of donor and acceptor membranes while the reaction volume was increased (50–1,000 μl), as indicated. The concentration of ATP (plus regeneration components) and cytosol was equivalent in all reactions. (B) Maturation kinetics. A standard 50-μl reaction (see Fig. 3) was scaled up fivefold, incubated at 25°C, and aliquots were removed at the indicated times. (C) Temperature dependence. Standard 50-μl reactions were incubated at the indicated temperatures. All reactions were immunoprecipitated for CPY, subjected to SDS-PAGE, and autoradiography.
Figure 5
The cell-free assay reconstitutes intercompartmental transport between donor and acceptor membranes. (A) Strategy for using yeast strains deleted for processing protease genes to test for intercompartmental transport in vitro (see Results for details). (B) Processing of CPY in intact spheroplasts from wild-type strains and strains deleted for the proteinase A gene (pep4Δ). Yeast spheroplasts with the indicated genotypes were radiolabeled for 5 min with Tran35S-label (0 min) and chased with unlabeled methionine and cysteine for 60 min. After each time point, the cells were lysed, immunoprecipitated for CPY, subjected to SDS-PAGE, and autoradiography. (C) Cell-free assays with donor and acceptor membranes from wild-type strains and strains deleted for the proteinase A gene (pep4Δ). Donor membranes from radiolabeled, and acceptor membranes from nonradiolabeled yeast spheroplasts were prepared as previously described (Fig. 3) from strains with the indicated genotypes. Standard reaction conditions (Fig. 3) were used to incubate all combinations of donor and acceptor membranes (with and without ATP and cytosol), as indicated. All reactions were immunoprecipitated for CPY, subjected to SDS-PAGE, and autoradiography.
Figure 6
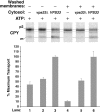
Cytosolic extracts from a vps33 null strain are deficient in stimulating intercompartmental transport in the cell-free system. Radiolabeled donor membranes and nonradiolabeled acceptor membranes were prepared from wild-type yeast spheroplasts (SEY6210). Standard reaction conditions (Fig. 3) were used to incubate the donor and acceptor membranes with ATP (plus regeneration components) and cytosol (5 mg/ml) from the VPS33 or vps33Δ strains, as indicated. The reactions in lanes 4–6 contained donor and acceptor membranes that had been washed once with lysis buffer (similar to Fig. 3, lanes 10 and 11) while the reactions in lanes 1–3 contained unwashed membranes. The bar graph depicts the average transport efficiency from three independent determinations and is normalized to the percent of maximal transport.
Figure 7
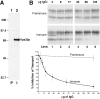
Polyclonal IgG against Vps33p inhibits cell-free reconstitution of intercompartmental transport between donor and acceptor membranes. (A) Specificity of the anti-Vps33p serum. Whole yeast cells (SEY6210) containing a plasmid with the VPS33 gene under control of the GPD1 promoter (pGPD426-33) were radiolabeled for 10 min (Tran35S-label) and chased (methionine and cysteine) for 30 min. After cell lysis, the extracts were immunoprecipitated with preimmune (P, lane 1) or immune (I, lane 2) serum against Vps33p, subjected to SDS-PAGE, and autoradiography. The positions of molecular weight standards are given in kD. (B) Purified immune IgG inhibits cell-free transport of CPY. Protein A–Sepharose was used to purify IgG from both preimmune and immune rabbit serum. Different amounts (as indicated) of the purified IgG were used in standard cell-free reactions with donor and acceptor membranes. All components of the reactions were added and incubated on ice with the indicated amounts of preimmune and immune IgG for 15 min before the standard 60-min incubation at 25°C. All reactions were immunoprecipitated for CPY, subjected to SDS-PAGE, and autoradiography.
Figure 8
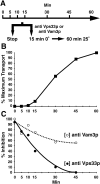
The function of Vps33p is required early during the cell-free reconstitution system and precedes the function of Vam3p. (A) Experimental strategy. (B) Transport kinetics. A standard 50-μl reaction (see Fig. 3) was scaled up, incubated at 25°C, and then aliquots were removed and stopped at the indicated times. (C) Antibody inhibition. Aliquots from the same reactions as in A were also removed and received either 128 μg of immune IgG against Vps33p or 5 μl of antiserum against Vam3p (as indicated), incubated 15 min on ice, and then shifted to 25°C for 60 min. All reactions were immunoprecipitated for CPY, subjected to SDS-PAGE, and autoradiography. Nonimmune serum was added to control for the effect of anti-Vam3p serum at each time point and did not inhibit the assay. A 2nd degree polynomial was used to fit the curve for the inhibition data points (C).
Figure 9
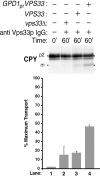
Biochemical reconstitution of Vps33p-dependent transport between donor and acceptor membranes. Radiolabeled donor membranes and nonradiolabeled acceptor membranes were prepared from wild-type yeast spheroplasts (Fig. 1 A). The donor and acceptor membranes were incubated for 15 min at 0°C with 128 μg of anti Vps33p IgG. Cytosol (5 mg/ml) was then added from a vps33Δ (lane 2), a VPS33 (lane 3), or a VPS33 strain containing pGPDHIS633-2 (lane 4), and incubated at 25°C for 60 min (as indicated). All reactions were immunoprecipitated for CPY, subjected to SDS-PAGE, and autoradiography. The bar graph depicts average transport efficiency from three independent determinations and normalized to the percent of maximal transport.
Similar articles
- In vitro reconstitution of intercompartmental protein transport to the yeast vacuole.
Vida TA, Graham TR, Emr SD. Vida TA, et al. J Cell Biol. 1990 Dec;111(6 Pt 2):2871-84. doi: 10.1083/jcb.111.6.2871. J Cell Biol. 1990. PMID: 2269659 Free PMC article. - A cell-free system for reconstitution of transport between prevacuolar compartments and vacuoles in Saccharomyces cerevisiae.
Vida TA. Vida TA. Methods Mol Biol. 2008;457:41-57. doi: 10.1007/978-1-59745-261-8_4. Methods Mol Biol. 2008. PMID: 19066018 - Yeast vacuolar proenzymes are sorted in the late Golgi complex and transported to the vacuole via a prevacuolar endosome-like compartment.
Vida TA, Huyer G, Emr SD. Vida TA, et al. J Cell Biol. 1993 Jun;121(6):1245-56. doi: 10.1083/jcb.121.6.1245. J Cell Biol. 1993. PMID: 8509446 Free PMC article. - Vacuolar sorting. Tracking down an elusive receptor.
Chapman RE. Chapman RE. Curr Biol. 1994 Nov 1;4(11):1019-22. doi: 10.1016/s0960-9822(00)00231-1. Curr Biol. 1994. PMID: 7874484 Review. - Novel pathways, membrane coats and PI kinase regulation in yeast lysosomal trafficking.
Burd CG, Babst M, Emr SD. Burd CG, et al. Semin Cell Dev Biol. 1998 Oct;9(5):527-33. doi: 10.1006/scdb.1998.0255. Semin Cell Dev Biol. 1998. PMID: 9835640 Review.
Cited by
- Assortment of phosphatidylinositol 3-kinase complexes--Atg14p directs association of complex I to the pre-autophagosomal structure in Saccharomyces cerevisiae.
Obara K, Sekito T, Ohsumi Y. Obara K, et al. Mol Biol Cell. 2006 Apr;17(4):1527-39. doi: 10.1091/mbc.e05-09-0841. Epub 2006 Jan 18. Mol Biol Cell. 2006. PMID: 16421251 Free PMC article. - Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae.
Kihara A, Noda T, Ishihara N, Ohsumi Y. Kihara A, et al. J Cell Biol. 2001 Feb 5;152(3):519-30. doi: 10.1083/jcb.152.3.519. J Cell Biol. 2001. PMID: 11157979 Free PMC article. - Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network.
Kihara A, Kabeya Y, Ohsumi Y, Yoshimori T. Kihara A, et al. EMBO Rep. 2001 Apr;2(4):330-5. doi: 10.1093/embo-reports/kve061. EMBO Rep. 2001. PMID: 11306555 Free PMC article. - Lem2 and Lnp1 maintain the membrane boundary between the nuclear envelope and endoplasmic reticulum.
Hirano Y, Kinugasa Y, Osakada H, Shindo T, Kubota Y, Shibata S, Haraguchi T, Hiraoka Y. Hirano Y, et al. Commun Biol. 2020 Jun 1;3(1):276. doi: 10.1038/s42003-020-0999-9. Commun Biol. 2020. PMID: 32483293 Free PMC article. - Heterologous expression and characterization of Schizosaccharomyces pombe vacuolar carboxypeptidase Y in Saccharomyces cerevisiae.
Takegawa K, Tokudomi S, Bhuiyan MS, Tabuchi M, Fujita Y, Iwaki T, Utsumi S, Tanaka N. Takegawa K, et al. Curr Genet. 2003 Feb;42(5):252-9. doi: 10.1007/s00294-002-0357-0. Epub 2002 Dec 14. Curr Genet. 2003. PMID: 12589464
References
- Baker D., Schekman R. Reconstitution of protein transport using broken yeast spheroplasts. Methods Cell Biol. 1989;31:127–141. - PubMed
- Balch W.E. Biochemistry of interorganelle transport. A new frontier in enzymology emerges from versatile in vitro model systems. J. Biol. Chem. 1989;264:16965–16968. - PubMed
- Balch W.E., Beckers C.J., Keller D.S. Reconstitution of protein transport from the endoplasmic reticulum to the Golgi using a cell free system. Prog. Clin. Biol. Res. 1988;270:333–342. - PubMed
- Balch W.E., Dunphy W.G., Braell W.A., Rothman J.E. Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell. 1984;39:405–416. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases