Drosophila roadblock and Chlamydomonas LC7: a conserved family of dynein-associated proteins involved in axonal transport, flagellar motility, and mitosis - PubMed (original) (raw)
. 1999 Jul 12;146(1):165-80.
Affiliations
- PMID: 10402468
- PMCID: PMC2199740
Drosophila roadblock and Chlamydomonas LC7: a conserved family of dynein-associated proteins involved in axonal transport, flagellar motility, and mitosis
A B Bowman et al. J Cell Biol. 1999.
Abstract
Eukaryotic organisms utilize microtubule-dependent motors of the kinesin and dynein superfamilies to generate intracellular movement. To identify new genes involved in the regulation of axonal transport in Drosophila melanogaster, we undertook a screen based upon the sluggish larval phenotype of known motor mutants. One of the mutants identified in this screen, roadblock (robl), exhibits diverse defects in intracellular transport including axonal transport and mitosis. These defects include intra-axonal accumulations of cargoes, severe axonal degeneration, and aberrant chromosome segregation. The gene identified by robl encodes a 97-amino acid polypeptide that is 57% identical (70% similar) to the 105-amino acid Chlamydomonas outer arm dynein-associated protein LC7, also reported here. Both robl and LC7 have homology to several other genes from fruit fly, nematode, and mammals, but not Saccharomyces cerevisiae. Furthermore, we demonstrate that members of this family of proteins are associated with both flagellar outer arm dynein and Drosophila and rat brain cytoplasmic dynein. We propose that roadblock/LC7 family members may modulate specific dynein functions.
Figures
Figure 1
The robl genomic interval. (A) A diagrammatic map of the five genes identified in the genomic region around roadblock (accession number AF141921). The entire region has been sequenced and cDNAs have been obtained for robl and genes 1 and 4. Gene 3 is a roadblock-like region, which is likely a pseudogene as it lacks any identifiable start codon. The two partially overlapping deficiencies robl l(2)k10408 and robl c identify the roadblock genomic region, dotted lines correspond to regions missing in deficiencies. The EMS mutant robl z deleted a small region in one of these genes allowing us to identify it as robl. The genomic rescue region shown completely rescues robl z/robl l(2)k10408. (B) The genomic sequence of the region encoding roadblock (corresponding to nucleotides 7,751–8,214 of genomic interval illustrated in A). Uppercase characters show the protein coding sequence that is translated below for each codon; lowercase characters are used to show the 5′-UTR, introns 1 and 2, and 3′-UTR. The EMS mutant robl z has a 193-bp deletion that is represented by bold characters. The deletion extends from intron 2 into the COOH terminus encoding exon 3, removing the intron's conserved branch point sequence that is underlined. Since robl z is a recessive neomorphic allele, a partially functional or aberrant protein is likely made. Reverse transcriptase–PCR analysis of robl z indicates that splicing of mutant intron 2 does not occur (data not shown). However, the mutant transcript maintains the correct reading frame through the remainder of intron 2 and exon 3. The resulting robl z protein would have an internal deletion of 54 residues (IPVKST…HEIMVA) replaced by a 12-residue insertion (GWFNCTSVCAKI) from the remainder of intron 2.
Figure 2
Molecular analysis of LC7 from the Chlamydomonas outer dynein arm. (A) Two tryptic peptides from outer arm dynein LC7 were completely sequenced, yielding a total of 26 residue assignments. The actual mass of each peptide is in agreement with the calculated mass once methionine oxidation of the upper peptide is incorporated. (B) DNA and predicted protein sequence for the Chlamydomonas LC7 cDNA clone. Both peptide sequences are found in the coding region (26/26 residues correct). These sequences are indicated in bold type and are contiguous in the primary structure. The polyadenylation signal is underlined. This sequence is available in the NCBI GenBank (accession number AF140239). (C) Southern blot of genomic DNA from Chlamydomonas strain S1D2 digested with BamHI, PstI, PvuII, and SmaI and probed with the full-length LC7 cDNA. The data suggest that there is a single gene for LC7 in Chlamydomonas. (D) Northern blot analysis of RNA from nondeflagellated cells and from those actively regenerating flagella (30′ postDF). A single message of ∼0.95 kb that is induced in regenerating cells is evident.
Figure 3
LC7 is a component of the outer dynein arm. (A) Flagellar axonemes were prepared from wild-type Chlamydomonas (WT) and from mutants lacking the outer arm (oda9), inner arm I1 (ida1, ida2, and ida3), and inner arms I2/3 (ida4). Samples were electrophoresed in a 5–15% acrylamide gradient gel and either stained with Coomassie blue (upper panel) or blotted to nitrocellulose and probed with the R7178 antibody to detect LC7. The LC7 protein is highly reduced only in the strain lacking outer dynein arms. (B) A high salt extract of wild-type axonemes was sedimented through a 5–20% sucrose gradient and fractions electrophoresed in 5–15% acrylamide gels. Fraction 1 is the bottom of the gradient. The upper panel shows the gel stained with Coomassie blue, the lower panel is an immunoblot probed with R7178 to detect the LC7 protein. All the salt extractable LC7 protein precisely comigrates at ∼18 S with components of the outer dynein arm (e.g., IC1 and IC2).
Figure 4
A large family of robl/LC7-like proteins. BLAST analysis has identified several mammalian ESTs, Drosophila genes, and a gene from C. elegans that are highly homologous to robl/LC7. (A) A dendrogram of the robl/LC7-like family members identified is shown. This dendrogram was generated using the GCG pileup command. We identified at least five Drosophila roadblock-like genes by searching the BDGP-derived ESTs and genomic sequences. Previously unidentified genes have been designated by their cytological location determined by BDGP. Also, two classes of robl/LC7-like genes have been identified as mammalian ESTs (identified by a representative EST accession number). (B) An alignment of the protein family is shown. The alignment was generated by the same GCG pileup command as an MSF file. Boxshade was used to illustrate aligned amino acid identity (dark shaded residues) and similarity (light shaded residues).
Figure 5
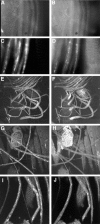
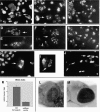
Coimmunostaining of larval segmental nerves for (left column) SYT and (right column) ChAT revealed distal axonal accumulations of synaptic cargo in robl mutants. (A and B) In wild type, there is only a low background staining observed. (C and D) Segmental nerves from robl z hemizygotes have accumulations of SYT and ChAT. (E and F) In robl z hemizygotes the segmental nerves at the anterior, coming off of the ventral ganglion (located at the bottom left quadrant in images E and F), show a decreased frequency of SYT accumulations but an increased frequency of ChAT accumulations when compared with more posterior regions. (G and H) The segmental nerves located at the posterior show an increased frequency of SYT accumulations with a corresponding decreased frequency of ChAT accumulations. (I and J) Segmental nerves from robl null homozygotes (robl k/robl k) show abundant accumulations of SYT and ChAT.
Figure 6
Transmission EM cross-sections of robl mutant third instar larval segmental nerves revealed two classes of axonal cargo accumulations and severe axonal loss and nerve degeneration. (A) Nerves from robl z hemizygous larvae had axons that swelled with transported material (dashed circles) and showed a loss of axons and nerve degeneration (area designated within arrows). (B) Axons from wild-type nerves do not show this swelled dense membranous axonal morphology. Two classes of axonal accumulations were observed in robl z hemizygotes: (C) small single component (small clear vesicles) axonal accumulations and (D) larger multi-component axonal accumulations. (E) Severely sluggish robl z hemizygous larvae showed increased axonal loss and degeneration. (F) All robl z homozygous larvae consistently showed a high degree of axonal loss and nerve degeneration. Bars: (A, B, E, and F) 500 nm; (C and D) 200 nm.
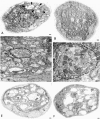
Figure 7
Severe mitotic defects were revealed in robl mutants examined by third instar larval brain squash analysis. Examples of typical wild-type mitotic figures are shown (designated by arrows): (A) a normal prometaphase figure, (B) a normal metaphase figure, and (C) a normal anaphase figure. Multiple abnormal mitotic figures are observed in robl z hemizygotes, including (designated by arrows): (D and E) aneuploid figures, (F) aneuploid figures with hypercondensed chromosomes, and (G) mutant anaphase figures with hypercondensed chromosomes disorganized around the presumptive poles. Mitotic figures are infrequently found in robl z homozygotes such as (designated by arrows): (H) a mutant anaphase structure with severe chromosome bridging, (I) another anaphase structure with a lagging chromosome and a single chromosome bridge, and (J) apparent telophase bridging. (K) The mitotic index of robl z hemizygotes is about five times that of wild type, the error bars indicate the SEM. robl z hemizygotes are late pupal lethal, when dissected from their pupal cases they show (L) rough pupal eyes. (M) A wild-type pupal eye is shown for comparison.
Figure 8
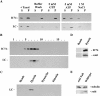
A robl/LC7-like protein is present in cytoplasmic dynein. (A) Western blot analysis was performed on samples from the fractionation of a rat brain homogenate. Blots were probed with mAb 74-1 and the R7178 rabbit polyclonal to detect IC74 of cytoplasmic dynein and the M r ∼12,000 robl/LC7-like protein, respectively. (B) Rat brain proteins eluted from microtubules with ATP were sedimented in a 5–20% sucrose gradient. Samples were probed with the 74-1 and R7178 antibodies. The robl/LC7-like protein precisely comigrates with IC74 of cytoplasmic dynein. (C) Rat brain homogenate immunoprecipitates of cytoplasmic dynein (antibody 74-1), kinesin (antibody H-2), dynactin (antibody 50-1), and a bead control were probed with the R7178 antibody. The robl/LC7-like protein is detectable only in the cytoplasmic dynein (IC74) sample. (D) Drosophila embryo immunoprecipitates of cytoplasmic dynein (antibody 74-1) or the bead control were probed with the 6883 anti-robl antibody and 74-1 anti-IC74 antibody. The roadblock protein was only precipitated in the cytoplasmic dynein (IC74) sample. (E) Equally loaded Drosophila robl null and wild-type larval homogenates were probed with the 6883 anti-robl antibody and with the 3A5 anti-tubulin antibody. The roadblock protein is undetectable in the robl null larvae, whereas tubulin is detected at about equal levels in null and wild-type larvae.
Figure 9
Summary of dynein-associated proteins. (A) A complete table of outer dynein arm–associated light chains is shown. The nominal mass refers to the M r determined by SDS-PAGE analysis; actual predicted mass of the proteins is given parenthetically. Previous work has elucidated biochemical interactions amongst these proteins. (B) A model of cytoplasmic dynein organization is shown. The cytoplasmic dynein particle is built around two heavy chains that form the stems and globular heads of the complex. Associated with the stems are a series of accessory proteins including: two IC74 intermediate chains that mediate dynein–dynactin interactions, two copies of the Tctex1 light chain (or of the related rp3 protein), one dimer of the highly conserved 10 kD/LC8 DLC (LC8 dimer), and a 22-kD polypeptide (the location of which is speculative). The present study indicates that cytoplasmic dynein also contains a robl/LC7-like protein that, by analogy with flagellar outer arm dynein, is located at the base of the dynein particle. A table of known proteins associated with cytoplasmic dynein is given; the majority are conserved in axonemal dyneins.
Similar articles
- The LC7 light chains of Chlamydomonas flagellar dyneins interact with components required for both motor assembly and regulation.
DiBella LM, Sakato M, Patel-King RS, Pazour GJ, King SM. DiBella LM, et al. Mol Biol Cell. 2004 Oct;15(10):4633-46. doi: 10.1091/mbc.e04-06-0461. Epub 2004 Aug 10. Mol Biol Cell. 2004. PMID: 15304520 Free PMC article. - The crystal structure of dynein intermediate chain-light chain roadblock complex gives new insights into dynein assembly.
Hall J, Song Y, Karplus PA, Barbar E. Hall J, et al. J Biol Chem. 2010 Jul 16;285(29):22566-75. doi: 10.1074/jbc.M110.103861. Epub 2010 May 15. J Biol Chem. 2010. PMID: 20472935 Free PMC article. - Dynein light chains of the Roadblock/LC7 group belong to an ancient protein superfamily implicated in NTPase regulation.
Koonin EV, Aravind L. Koonin EV, et al. Curr Biol. 2000 Nov 2;10(21):R774-6. doi: 10.1016/s0960-9822(00)00774-0. Curr Biol. 2000. PMID: 11084347 No abstract available. - Keeping an eye on I1: I1 dynein as a model for flagellar dynein assembly and regulation.
Wirschell M, Hendrickson T, Sale WS. Wirschell M, et al. Cell Motil Cytoskeleton. 2007 Aug;64(8):569-79. doi: 10.1002/cm.20211. Cell Motil Cytoskeleton. 2007. PMID: 17549744 Review. - Regulation of dynein-driven microtubule sliding by an axonemal kinase and phosphatase in Chlamydomonas flagella.
Habermacher G, Sale WS. Habermacher G, et al. Cell Motil Cytoskeleton. 1995;32(2):106-9. doi: 10.1002/cm.970320207. Cell Motil Cytoskeleton. 1995. PMID: 8681389 Review.
Cited by
- Structure and Function of Dynein's Non-Catalytic Subunits.
Rao L, Gennerich A. Rao L, et al. Cells. 2024 Feb 11;13(4):330. doi: 10.3390/cells13040330. Cells. 2024. PMID: 38391943 Free PMC article. Review. - The genetics of axonal transport and axonal transport disorders.
Duncan JE, Goldstein LS. Duncan JE, et al. PLoS Genet. 2006 Sep 29;2(9):e124. doi: 10.1371/journal.pgen.0020124. PLoS Genet. 2006. PMID: 17009871 Free PMC article. Review. - The LC7 light chains of Chlamydomonas flagellar dyneins interact with components required for both motor assembly and regulation.
DiBella LM, Sakato M, Patel-King RS, Pazour GJ, King SM. DiBella LM, et al. Mol Biol Cell. 2004 Oct;15(10):4633-46. doi: 10.1091/mbc.e04-06-0461. Epub 2004 Aug 10. Mol Biol Cell. 2004. PMID: 15304520 Free PMC article. - Cytoplasmic dynein, the dynactin complex, and kinesin are interdependent and essential for fast axonal transport.
Martin M, Iyadurai SJ, Gassman A, Gindhart JG Jr, Hays TS, Saxton WM. Martin M, et al. Mol Biol Cell. 1999 Nov;10(11):3717-28. doi: 10.1091/mbc.10.11.3717. Mol Biol Cell. 1999. PMID: 10564267 Free PMC article. - The Drosophila tctex-1 light chain is dispensable for essential cytoplasmic dynein functions but is required during spermatid differentiation.
Li MG, Serr M, Newman EA, Hays TS. Li MG, et al. Mol Biol Cell. 2004 Jul;15(7):3005-14. doi: 10.1091/mbc.e04-01-0013. Epub 2004 Apr 16. Mol Biol Cell. 2004. PMID: 15090621 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous