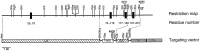Rabphilin knock-out mice reveal that rabphilin is not required for rab3 function in regulating neurotransmitter release - PubMed (original) (raw)
Rabphilin knock-out mice reveal that rabphilin is not required for rab3 function in regulating neurotransmitter release
O M Schlüter et al. J Neurosci. 1999.
Abstract
Rab3A and rab3C are GTP-binding proteins of synaptic vesicles that regulate vesicle exocytosis. Rabphilin is a candidate rab3 effector at the synapse because it binds to rab3s in a GTP-dependent manner, it is co-localized with rab3s on synaptic vesicles, and it dissociates with rab3s from the vesicles during exocytosis. Rabphilin contains two C(2) domains, which could function as Ca(2+) sensors in exocytosis and is phosphorylated as a function of stimulation. However, it is unknown what essential function, if any, rabphilin performs. One controversial question regards the respective roles of rab3s and rabphilin in localizing each other to synaptic vesicles: although rabphilin is mislocalized in rab3A knock-out mice, purified synaptic vesicles were shown to require rabphilin for binding of rab3A but not rab3A for binding of rabphilin. To test whether rabphilin is involved in localizing rab3s to synaptic vesicles and to explore the functions of rabphilin in regulating exocytosis, we have now analyzed knock-out mice for rabphilin. Mice that lack rabphilin are viable and fertile without obvious physiological impairments. In rabphilin-deficient mice, rab3A is targeted to synaptic vesicles normally, whereas in rab3A-deficient mice, rabphilin transport to synapses is impaired. These results show that rabphilin binds to vesicles via rab3s, consistent with an effector function of rabphilin for a synaptic rab3-signal. Surprisingly, however, no abnormalities in synaptic transmission or plasticity were observed in rabphilin-deficient mice; synaptic properties that are impaired in rab3A knock-out mice were unchanged in rabphilin knock-out mice. Our data thus demonstrate that rabphilin is endowed with the properties of a rab3 effector but is not essential for the regulatory functions of rab3 in synaptic transmission.
Figures
Fig. 1.
Model of Ca2+-binding proteins on synaptic vesicles: mode of rabphilin attachment to synaptic vesicles. A schematic view of the three known Ca2+-binding proteins of synaptic vesicles is shown: synaptotagmins I and II bind multiple Ca2+ ions via two C2 domains (Südhof and Rizo, 1996); synapsins I and III are directly regulated by Ca2+(Hosaka and Südhof, 1998a,b); and rabphilin, which also has two C2 domains that probably bind Ca2+. Two models for the role of the GTP-dependent binding of rabphilin to rab3s are presented: the hypothesis of Stahl et al. (1996) that rab3A is bound to vesicles and serves to recruit rabphilin (Model 1); or the hypothesis of Shirataki et al. (1994) that rabphilin is primarily bound to vesicles and is responsible for the vesicle-specific binding of rab3s (Model 2).
Fig. 2.
Partial structure of the murine rabphilin gene and gene targeting strategy for the rabphilin knock-out. The restiction map on top indicates positions of enzyme sites (N, _Nhe_I; B,_Bam_HI; H, _Hin_dIII;E, _Eco_RI; K,_Kpn_I; X, _Xho_I;Xb, _Xba_I; C, Cla_I;S, Sal_I). Exons are shown as black boxes_with the corresponding residue numbers. In the targeting vector on the_bottom, the long arm (LA) and short arm (SA) for recombination are marked by hatched boxes, and the gene cassettes for positive selection (neo, neomycin resistance gene) and for negative selection (TK, thymidine kinase gene) are marked by_open boxes. Positions of oligonucleotide primers for PCR genotyping are indicated by arrows, and the location of the outside probe used for Southern blotting is indicated by_OP. Note that homologous recombination replaces genomic sequences containing two exons encoding residues 74–146 with the neomycin gene.
Fig. 3.
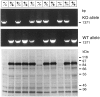
PCR and immunoblotting analysis of wild-type and mutant mice. The top panels display a PCR analysis of DNA of the offspring from a heterozygous mating of rabphilin mutant mice. Brain proteins from the same animals were analyzed on the_bottom panel_ with anti-rabphilin serum (Li et al., 1994). To show the absence of any additional bands in the mutant mice, a long exposure is shown. The genotype of the animals deduced from the analysis is indicated on top. KO, Knock-out; WT, wild-type.
Fig. 4.
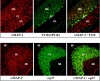
Confocal immunofluorescence analysis of brain sections from rabphilin knock-out mice. Frozen sections (5 μm) from the cerebellum (top panel) and hippocampus (bottom panel) of adult homozygous mutant mice were double-labeled with a monoclonal antibody to MAP-2 (MAP-2; red) and a polyclonal antibody to the IP3 receptor (T210; _green_in top panel) or synaptophysin II (p37; green in bottom panel). Points of colocalization of the signals are shown in the right panels in yellow. The granule cell layer (GL), the Purkinje cell layer (PL), and the molecular layer (ML) of the cerebellum and the stratum radiatum (SR), the stratum lucidum (SL), and the stratum pyramidale (SP) of the hippocampal CA3 region are marked.
Fig. 5.
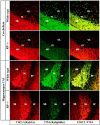
Comparative immunofluorescence analysis of rab3A and rabphilin in frozen sections from wild-type and rabphilin knock-out mice. Frozen sections (5 μm) were double-labeled with a monoclonal antibody against rab3A (Cl42.2; red_signal) and a polyclonal antibody against rabphilin (I734; green signal). Sections were viewed with a confocal microscope; signal colocalization is shown in_yellow. GL, Granule cell layer;PL, Purkinje cell layer; ML, molecular layer; SR, stratum radiatum; SL, stratum lucidum; SP, stratum pyramidale.
Fig. 6.
Immunofluorescence analysis of retina sections from wild-type and rabphilin knock-out mice. Frozen sections (5 μm) from retina were double-labeled with a monoclonal antibody to rab3A (Cl42.2; red) and a polyclonal antibody to rabphilin (I734; green). Colocalization of the two proteins is shown in yellow. The inner plexiform layer (IPL) and outer plexiform layer (OPL) are identified.
Fig. 7.
Subcellular fractionation of wild-type and rabphilin knock-out brains. Brain cortex from rabphilin-deficient (−/−) or wild-type brains (+/+) were homogenized (H) and fractionated into nuclear pellets (P1), crude synaptosomes (P2), and a high-speed pellet (P3). The synaptosomes were then used to derive crude synaptic plasma membranes (LP1), crude synaptic vesicles (LP2), cytosol (S3), synaptosomal cytosol (LS2), and synaptic plasma membranes (SPM). The fractions were analyzed by SDS-PAGE and immunoblotting with the indicated antibodies:Cl54.1, NMDA-receptor 1; Cl81.1, GDI; α_p37_, synaptophysin II; Cl42.2, rab3A;Cl69.1, synaptobrevin 2.
Fig. 8.
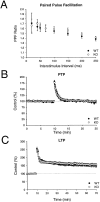
Short-term synaptic plasticity and LTP are normal at mossy fiber synapses in the hippocampal CA3 region in rabphilin knock-out mice. A 1, A 2, Magnitude of paired pulse facilitation at 20 msec (A 1) and 40 msec (A 2) interstimulus intervals in wild type (WT) and rabphilin knock-out mice (KO).B 1, B 2, Frequency facilitation induced by increasing the stimulation rate from 0.1 to 0.33 Hz (B 1) or 0.2 Hz (B 2) in wild-type and rabphilin knock-out mice.C, Induction of LTP by a 25 Hz, 5 sec tetanus (arrow) in wild-type and rabphilin knock-out mice.
Fig. 9.
Short-term synaptic plasticity and LTP are normal at excitatory synapses in the hippocampal CA1 region in rabphilin knock-out mice. A, Paired pulse facilitation as a function of interstimulus interval in wild-type and rabphilin knock-out mice. B, Time course of posttetanic potentiation elicited by a 100 Hz, 1 sec tetanus in wild-type and rabphilin knock-out mice. C, Induction of LTP by three 100 Hz, 1 sec tetani in wild-type and rabphilin knock-out mice.
Fig. 10.
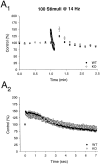
Synaptic depression is normal at excitatory synapses in the CA1 region of rabphilin knock-out mice. Time course of the effects of a stimulus train (100 stimuli at 14 Hz) is shown on a long (A 1) and short (A 2) time scale in wild type (WT) and rabphilin knock-out (KO) mice.
Similar articles
- Synaptic targeting of rabphilin-3A, a synaptic vesicle Ca2+/phospholipid-binding protein, depends on rab3A/3C.
Li C, Takei K, Geppert M, Daniell L, Stenius K, Chapman ER, Jahn R, De Camilli P, Südhof TC. Li C, et al. Neuron. 1994 Oct;13(4):885-98. doi: 10.1016/0896-6273(94)90254-2. Neuron. 1994. PMID: 7946335 - Rab3 reversibly recruits rabphilin to synaptic vesicles by a mechanism analogous to raf recruitment by ras.
Stahl B, Chou JH, Li C, Südhof TC, Jahn R. Stahl B, et al. EMBO J. 1996 Apr 15;15(8):1799-809. EMBO J. 1996. PMID: 8617225 Free PMC article. - The role of Rab3A in neurotransmitter release.
Geppert M, Bolshakov VY, Siegelbaum SA, Takei K, De Camilli P, Hammer RE, Südhof TC. Geppert M, et al. Nature. 1994 Jun 9;369(6480):493-7. doi: 10.1038/369493a0. Nature. 1994. PMID: 7911226 - Rab3A-rabphilin-3A system in neurotransmitter release.
Sasaki T, Shirataki H, Nakanishi H, Takai Y. Sasaki T, et al. Adv Second Messenger Phosphoprotein Res. 1997;31:279-94. doi: 10.1016/s1040-7952(97)80025-0. Adv Second Messenger Phosphoprotein Res. 1997. PMID: 9344258 Review. No abstract available. - Rab3A small GTP-binding protein in Ca(2+)-dependent exocytosis.
Takai Y, Sasaki T, Shirataki H, Nakanishi H. Takai Y, et al. Genes Cells. 1996 Jul;1(7):615-32. doi: 10.1046/j.1365-2443.1996.00257.x. Genes Cells. 1996. PMID: 9078389 Review.
Cited by
- Rabphilin potentiates soluble N-ethylmaleimide sensitive factor attachment protein receptor function independently of rab3.
Staunton J, Ganetzky B, Nonet ML. Staunton J, et al. J Neurosci. 2001 Dec 1;21(23):9255-64. doi: 10.1523/JNEUROSCI.21-23-09255.2001. J Neurosci. 2001. PMID: 11717359 Free PMC article. - Synaptic state-dependent functional interplay between postsynaptic density-95 and synapse-associated protein 102.
Bonnet SA, Akad DS, Samaddar T, Liu Y, Huang X, Dong Y, Schlüter OM. Bonnet SA, et al. J Neurosci. 2013 Aug 14;33(33):13398-409. doi: 10.1523/JNEUROSCI.6255-11.2013. J Neurosci. 2013. PMID: 23946397 Free PMC article. - cAMP-Dependent Synaptic Plasticity at the Hippocampal Mossy Fiber Terminal.
Shahoha M, Cohen R, Ben-Simon Y, Ashery U. Shahoha M, et al. Front Synaptic Neurosci. 2022 Apr 4;14:861215. doi: 10.3389/fnsyn.2022.861215. eCollection 2022. Front Synaptic Neurosci. 2022. PMID: 35444523 Free PMC article. Review. - The Role of Rab Proteins in Parkinson's Disease Synaptopathy.
Bellucci A, Longhena F, Spillantini MG. Bellucci A, et al. Biomedicines. 2022 Aug 10;10(8):1941. doi: 10.3390/biomedicines10081941. Biomedicines. 2022. PMID: 36009486 Free PMC article. Review. - Searching for molecular players differentially involved in neurotransmitter and neuropeptide release.
Xu T, Xu P. Xu T, et al. Neurochem Res. 2008 Oct;33(10):1915-9. doi: 10.1007/s11064-008-9648-2. Epub 2008 Apr 10. Neurochem Res. 2008. PMID: 18401709 Review.
References
- Arribas M, Regazzi R, Garcia E, Wollheim CB, De Camilli P. The stimulatory effect of rabphilin 3a on regulated exocytosis from insulin-secreting cells does not require an association-dissociation cycle with membranes mediated by rab3. Eur J Cell Biol. 1997;74:209–216. - PubMed
- Betz A, Ashery U, Rickmann M, Augustin I, Neher E, Südhof TC, Rettig J, Brose N. Munc13–1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron. 1998;21:123–136. - PubMed
- Brose N, Huntley GW, Stern-Bach Y, Sharma G, Morrison JH, Heinemann S. Differential assembly of coexpressed glutamate receptor subunits in neurons of rat cerebral cortex. J Biol Chem. 1994;269:16780–16784. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous