The Epstein-Barr virus oncoprotein latent membrane protein 1 engages the tumor necrosis factor receptor-associated proteins TRADD and receptor-interacting protein (RIP) but does not induce apoptosis or require RIP for NF-kappaB activation - PubMed (original) (raw)
The Epstein-Barr virus oncoprotein latent membrane protein 1 engages the tumor necrosis factor receptor-associated proteins TRADD and receptor-interacting protein (RIP) but does not induce apoptosis or require RIP for NF-kappaB activation
K M Izumi et al. Mol Cell Biol. 1999 Aug.
Free PMC article
Abstract
A site in the Epstein-Barr virus (EBV) transforming protein LMP1 that constitutively associates with the tumor necrosis factor receptor 1 (TNFR1)-associated death domain protein TRADD to mediate NF-kappaB and c-Jun N-terminal kinase activation is critical for long-term lymphoblastoid cell proliferation. We now find that LMP1 signaling through TRADD differs from TNFR1 signaling through TRADD. LMP1 needs only 11 amino acids to activate NF-kappaB or synergize with TRADD in NF-kappaB activation, while TNFR1 requires approximately 70 residues. Further, LMP1 does not require TRADD residues 294 to 312 for NF-kappaB activation, while TNFR1 requires TRADD residues 296 to 302. LMP1 is partially blocked for NF-kappaB activation by a TRADD mutant consisting of residues 122 to 293. Unlike TNFR1, LMP1 can interact directly with receptor-interacting protein (RIP) and stably associates with RIP in EBV-transformed lymphoblastoid cell lines. Surprisingly, LMP1 does not require RIP for NF-kappaB activation. Despite constitutive association with TRADD or RIP, LMP1 does not induce apoptosis in EBV-negative Burkitt lymphoma or human embryonic kidney 293 cells. These results add a different perspective to the molecular interactions through which LMP1, TRADD, and RIP participate in B-lymphocyte activation and growth.
Figures
FIG. 1
Diagram of LMP1. The Flag epitope was introduced at the amino terminus (NH2). LMP1 residues 187, 231, 352, and 386 are marked. LMP1 constitutively aggregates in the plasma membrane and associates with TRAFs, TRADD, and RIP. TES1 aggregates TRAFs to mediate low-level NF-κB activation and initial B-lymphocyte growth transformation. TES2 aggregates RIP or TRADD, both of which associate with TRAFs to mediate high-level NF-κB activation and enable permanent LCL outgrowth.
FIG. 2
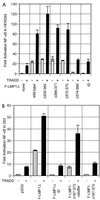
(A) Characterization of the LMP1 residues critical for engaging TRADD to synergistically activate NF-κB. Five million HEK293 cells were electroporated with 30 μg of pSG5 or TES2/CTAR2 vector F-LMP1Δ, 5 μg of TRADD vector where indicated, 2.5 μg of 3x-κB-L luciferase reporter, which has three copies of a major histocompatibility complex class I κB element and minimal_fos_ promoter, and 2.5 μg of glucokinase promoter/β-galactosidase reporter to monitor transfection efficiency; 18 h later, lysates were analyzed for luciferase (Promega) and β-galactosidase (Tropix) according to the manufacturers’ directions on an Opticomp I luminometer. The base wild-type F-LMP1Δ vector is deleted for residues 187 to 351. Further deletions are indicated. ID indicates mutation of the terminal residues Y384YD386 to ID. Standard error of means are reported. In data not shown, protein levels of TRADD or LMP1 mutants were equivalent in separate transfections as analyzed by Western immunoblotting. (B) The 11 terminal residues of LMP1 are sufficient to engage TRADD to synergistically activate NF-κB. F-LMP1 vectors deleted for residues 187 to 375 or with a short stuffer consisting of residues HGHLGASLQY inserted between residues 186 and 376 were electroporated into HEK293 cells and analyzed as described for panel A.
FIG. 3
LMP1 synergistically activates NF-κB with TRADD mutants that are unable to engage TRAFs or efficiently interact with the TNFR1 death domain. Five micrograms of full-length TRADD 1-312 vector DNA, 30 μg of TRADD 1-293 DNA (crippled for death domain association), 15 μg of TRADD 122-312 DNA (crippled for TRAF2 aggregation), or 15 μg of TRADD 122-293 DNA was cotransfected with 30 μg of pSG5 or TES2/CTAR2 vector F-LMP1Δ into HEK293 cells along with NF-κB and β-galactosidase reporter DNAs; analysis and reporting of data are as described for Fig. 2A. LMP1 and TRADD protein levels monitored by Western immunoblotting were equivalent between the individual transfections.
FIG. 4
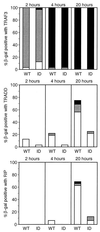
Yeast two-hybrid assay of LMP1 interaction with TRAF3, TRADD, or RIP monitored by β-galactosidase conversion of X-Gal.S. cerevisiae Y190 was transformed with pAS1 vectors expressing the Gal4 DBD fused to LMP1 amino acids 187 to 386 (wild type [WT]) or fused to LMP1 amino acids 187 to 386 with a mutation of Y384YD386 to ID (ID) and with pACT2 vectors expressing the Gal4 AD fused to TRAF3 (residues 312 to 568), TRADD (residues 1 to 312), or RIP (residues 1 to 671). Cotransformed yeast cells were selected on medium deficient in tryptophan and leucine; 32 colonies were individually transferred to filters, frozen, and thawed twice, incubated at 37°C with 1 mg of X-Gal per ml in buffer (100 mM sodium phosphate [pH 7.0], 10 mM KCl, 0.13 mM 2-mercaptoethanol), and monitored for blue-colored product. Intensity was scored as dark blue (■), blue (░⃞), or light blue (□).
FIG. 5
Coimmunoprecipitation of RIP or TRADD with F-LMP1. Proteins from LCLs (2.0 × 108 cells) infected with an EBV recombinant expressing F-LMP1 or wild-type LMP1 were solubilized by Dounce disruption in 0.5% Brij 58–100 mM NaCl–50 mM Tris (pH 7.2) and immunoprecipitated with a Flag-specific M2 affinity gel (Kodak). Precipitated proteins were Western blotted with antisera to RIP (Pharmingen), TRADD (Santa Cruz Biotechnology) or S12 monoclonal antibody to LMP1. Input lanes represent unfractionated cell proteins, unbound lanes represent proteins not precipitated with M2 affinity gel, and Imm Ppt lanes represent immunoprecipitated proteins. Percentages indicate fractions of total samples analyzed.
FIG. 6
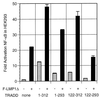
TRADD but not RIP synergistically activates NF-κB with LMP1. Five million HEK293 cells were electroporated with 3 μg of pSG5, TES2/CTAR2 vector F-LMP1Δ, or 6 μg of F-LMP1ΔID vector 3 μg of TRADD vector or 0.35 μg of RIP vector, and the NF-κB and β-galactosidase reporters used for Fig. 2A. Results were analyzed as described for Fig. 2A.
FIG. 7
LMP1 or LMP1 TES2/CTAR2 deleted for residues 187 to 351 activates NF-κB in RIP-positive SVT35 Jurkat cells and RIP-deficient 35.3.13 Jurkat cells. Fifteen million SVT35 or 35.3.13 cells were electroporated with 9 μg of pSG5 or the indicated amounts of F-LMP1 vector or TES2/CTAR2 vector F-LMP1Δ and with 1 μg of 3x-κB-L, an NF-κB-responsive luciferase reporter. After 18 h, cultures were divided in two. One-half was untreated, while the other half was stimulated with 200 nM phorbol myristate acetate (PMA) and 10 nM ionomycin for 4 h, at which time both samples were tested and compared for luciferase activity. The results are the means of relative light unit (RLU) emission from cells transfected with LMP1 vector divided by the RLU emission of cells further stimulated with PMA and ionomycin minus baseline values for pSG5 vector-transfected cultures. In SVT35 (RIP-positive) cells, unstimulated and maximally activated luciferase activities were 2,800 and 109,000 RLU, respectively (baseline 2.6% of maximum), whereas in 35.3.13 (RIP-negative) cells, unstimulated and maximally activated luciferase activities were 7,800 and 111,000 RLU, respectively (baseline 7.1% of maximum). Levels of F-LMP1 and F-LMP1Δ were monitored by Western immunoblotting and were equivalent at the same DNA dosage for both cell lines. Further, RIP expression in SVT35 and deficiency in 35.3.13 was confirmed by Western immunoblotting (data not shown).
FIG. 8
F-LMP1Δ and F-LMP1ΔID do not activate programmed cell death, whereas TRADD induces apoptosis. BJAB B-lymphoma cells were cotransfected with GFP vector pEGFP (Clontech) and with pSG5, TES2/CTAR2 vector F-LMP1Δ, F-LMP1ΔID vector, or TRADD vector. After transfection, dead cells were removed by gradient centrifugation. Viable cells were cultured for 10 h and then treated for 8 h with cycloheximide or left untreated. Next, cells were fixed in paraformaldehyde, permeabilized with ethanol, and stained for DNA with propidium iodide. DNA content in GFP-positive cells was quantitated by fluorescence-activated cytometry and plotted as propidium iodide fluorescence intensity (x axis) versus cell frequency (y axis). Apoptotic cells have hypodiploid DNA content (Ao) and are indicated by a line at the left and percentage at the right. Results shown are for cells that were not cycloheximide treated.
FIG. 9
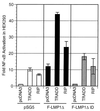
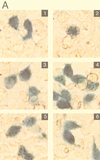
LMP1 and F-LMP1Δ do not activate apoptosis when NF-κB activation is blocked by the presence of a nondegradable IκBα (IκBα SSAA). HEK293 cells were cotransfected with pcDNA3 (panel 1), TRADD (panel 2), pcDNA3 LMP1 (panel 3), pcDNA3 LMP1 and IκBα SSAA (panel 4), TES2/CTAR2 vector pcDNA3 F-LMP1Δ (panel 5), or pcDNA3 F-LMP1Δ and IκBα SSAA (panel 6) and with β-galactosidase and NF-κB reporter DNAs. (A) Cells were fixed and then incubated in X-Gal prior to photography. The percentage of apoptotic, β-galactosidase-positive cells was 4% for pcDNA3 (panel 1), 57% for TRADD (panel 2), 8% for pcDNA3 LMP1 (panel 3), 3% for pcDNA3 LMP1 and IκBα SSAA (panel 4), 2% for pcDNA3 F-LMP1Δ (panel 5), and 3% for pcDNA3 F-LMP1Δ and IκBα SSAA (panel 6). (B) NF-κB activation as described for Fig. 2A. (C) Western immunoblot assay for LMP1 and IκBα SSAA. Equivalent amounts of protein were size separated in denaturing polyacrylamide gels, blotted to nitrocellulose, and probed with antibody M2 (Kodak) to F-LMP1Δ or Flag-tagged IκBα SSAA or antibody S12 to F-LMP1.
FIG. 9
LMP1 and F-LMP1Δ do not activate apoptosis when NF-κB activation is blocked by the presence of a nondegradable IκBα (IκBα SSAA). HEK293 cells were cotransfected with pcDNA3 (panel 1), TRADD (panel 2), pcDNA3 LMP1 (panel 3), pcDNA3 LMP1 and IκBα SSAA (panel 4), TES2/CTAR2 vector pcDNA3 F-LMP1Δ (panel 5), or pcDNA3 F-LMP1Δ and IκBα SSAA (panel 6) and with β-galactosidase and NF-κB reporter DNAs. (A) Cells were fixed and then incubated in X-Gal prior to photography. The percentage of apoptotic, β-galactosidase-positive cells was 4% for pcDNA3 (panel 1), 57% for TRADD (panel 2), 8% for pcDNA3 LMP1 (panel 3), 3% for pcDNA3 LMP1 and IκBα SSAA (panel 4), 2% for pcDNA3 F-LMP1Δ (panel 5), and 3% for pcDNA3 F-LMP1Δ and IκBα SSAA (panel 6). (B) NF-κB activation as described for Fig. 2A. (C) Western immunoblot assay for LMP1 and IκBα SSAA. Equivalent amounts of protein were size separated in denaturing polyacrylamide gels, blotted to nitrocellulose, and probed with antibody M2 (Kodak) to F-LMP1Δ or Flag-tagged IκBα SSAA or antibody S12 to F-LMP1.
References
- Ashkenazi A, Dixit V M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. - PubMed
- Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi J P, van Kooten C, Liu Y J, Rousset F, Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. - PubMed
- Beg A A, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. - PubMed
- Brodeur S R, Cheng G, Baltimore D, Thorley-Lawson D. Localization of the major NF-kappaB-activating site and the sole TRAF3 binding site of LMP-1 defines two distinct signaling motifs. J Biol Chem. 1997;272:19777–19784. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous