A set of independent selectable markers for transfection of the human malaria parasite Plasmodium falciparum - PubMed (original) (raw)
A set of independent selectable markers for transfection of the human malaria parasite Plasmodium falciparum
C B Mamoun et al. Proc Natl Acad Sci U S A. 1999.
Erratum in
- Proc Natl Acad Sci U S A 1999 Sep 14;96(19):10944
Abstract
Genomic information is rapidly accumulating for the human malaria pathogen, Plasmodium falciparum. Our ability to perform genetic manipulations to understand Plasmodium gene function is limited. Dihydrofolate reductase is the only selectable marker presently available for transfection of P. falciparum. Additional markers are needed for complementation and for expression of mutated forms of essential genes. We tested parasite sensitivity to different drugs for which selectable markers are available. Two of these drugs that were very effective as antiplasmodial inhibitors in culture, blasticidin and geneticin (G418), were selected for further study. The genes BSD, encoding blasticidin S deaminase of Aspergillus terreus, and NEO, encoding neomycin phosphotransferase II from transposon Tn 5, were expressed under the histidine-rich protein III (HRPIII) gene promoter and tested for their ability to confer resistance to blasticidin or G418, respectively. After transfection, blasticidin and G418-resistant parasites tested positive for plasmid replication and BSD or NEO expression. Cross-resistance assays indicate that these markers are independent. The plasmid copy number and the enzymatic activity depended directly on the concentration of the drug used for selection. These markers set the stage for new methods of functional analysis of the P. falciparum genome.
Figures
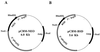
Figure 1
Maps of pCBM-BSD and pCBM-NEO vectors.
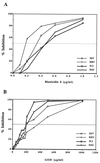
Figure 2
Inhibition of 3D7, HB3, W2, and Dd2 parasite clones as a function of blasticidin S (A) or G418 (B) concentrations.
Figure 3
(A) Detection of pCBM-BSD DNA from parasites selected on 1 μg/ml (BS1), 2 μg/ml (BS2), or 5 μg/ml (BS5) of blasticidin S. Genomic DNA (prepared 7 weeks posttransfection) and DNA from the original plasmid (pCBM-BSD) were restricted with _Sca_I (S, which cuts once in the vector) or _Sca_I + _Dpn_I (SD, with 15 pBluescript recognition sites), hybridized with a 32P-labeled pBluescript probe, and detected by autoradiography. A longer exposure of the autoradiograph is also shown. (B) Detection of pCBM-NEO DNA from parasites selected on 500 μg/ml G418 (G5). DNA was prepared as described for A. Untransfected 3D7 served as a control in both A and B.
Figure 4
Plasmid copy number in G3 and G5 clones (Upper) and BS1, BS2, and BS5 (Lower). By using the genome size of 3 × 107 bp, the amount of NEO (800 bp) or BSD (390 bp) signal that would represent one copy per P. falciparum genome was calculated; 0.5 μg of DNA from 3D7 and the various transformants were used for comparison.
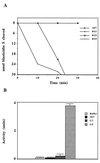
Figure 5
Enzyme assays. (A) BSD activity. Crude cell extracts from 3D7 alone or 3D7 transfected parasites selected on 1 μg/ml (BS1), 2 μg/ml (BS2), or 5 μg/ml (BS5) of blasticidin S were added to substrate in the assay buffer. Samples were taken at 0, 10, 20, and 30 min after incubation. The differential UV absorbance between the substrate (blasticidin S) and the deaminated product was recorded. (B) Neomycin phosphotransferase II activity. Crude cell extracts from 3D7 alone or 3D7 transfected parasites selected on 300 μg/ml (G3) or 500 (G5) μg/ml G418 were incubated with [γ-32P]ATP and kanamycin sulfate in the assay buffer at 37°C for 60 min. The buffer alone was used as a control.
Similar articles
- Rapid positive selection of stable integrants following transfection of Plasmodium falciparum.
Wang P, Wang Q, Sims PF, Hyde JE. Wang P, et al. Mol Biochem Parasitol. 2002 Aug 7;123(1):1-10. doi: 10.1016/s0166-6851(02)00105-6. Mol Biochem Parasitol. 2002. PMID: 12165384 - New selectable markers and single crossover integration for the highly versatile Plasmodium knowlesi transfection system.
Wel Av, Kocken CH, Pronk TC, Franke-Fayard B, Thomas AW. Wel Av, et al. Mol Biochem Parasitol. 2004 Mar;134(1):97-104. doi: 10.1016/j.molbiopara.2003.10.019. Mol Biochem Parasitol. 2004. PMID: 14747147 - Blasticidin S deaminase gene from Aspergillus terreus (BSD): a new drug resistance gene for transfection of mammalian cells.
Kimura M, Takatsuki A, Yamaguchi I. Kimura M, et al. Biochim Biophys Acta. 1994 Nov 22;1219(3):653-9. doi: 10.1016/0167-4781(94)90224-0. Biochim Biophys Acta. 1994. PMID: 7948022 - Assessment of copy number variation in genes related to drug resistance in Plasmodium vivax and Plasmodium falciparum isolates from the Brazilian Amazon and a systematic review of the literature.
Costa GL, Amaral LC, Fontes CJF, Carvalho LH, de Brito CFA, de Sousa TN. Costa GL, et al. Malar J. 2017 Apr 19;16(1):152. doi: 10.1186/s12936-017-1806-z. Malar J. 2017. PMID: 28420389 Free PMC article. Review. - Functional analysis of drug resistance in Plasmodium falciparum in the post-genomic era.
Cowman AF. Cowman AF. Int J Parasitol. 2001 Jul;31(9):871-8. doi: 10.1016/s0020-7519(01)00201-6. Int J Parasitol. 2001. PMID: 11406136 Review.
Cited by
- Investigations into the role of the Plasmodium falciparum SERCA (PfATP6) L263E mutation in artemisinin action and resistance.
Valderramos SG, Scanfeld D, Uhlemann AC, Fidock DA, Krishna S. Valderramos SG, et al. Antimicrob Agents Chemother. 2010 Sep;54(9):3842-52. doi: 10.1128/AAC.00121-10. Epub 2010 Jun 21. Antimicrob Agents Chemother. 2010. PMID: 20566762 Free PMC article. - Invasion by P. falciparum merozoites suggests a hierarchy of molecular interactions.
Baum J, Maier AG, Good RT, Simpson KM, Cowman AF. Baum J, et al. PLoS Pathog. 2005 Dec;1(4):e37. doi: 10.1371/journal.ppat.0010037. Epub 2005 Dec 16. PLoS Pathog. 2005. PMID: 16362075 Free PMC article. Review. - Overexpression of plasmepsin II and plasmepsin III does not directly cause reduction in Plasmodium falciparum sensitivity to artesunate, chloroquine and piperaquine.
Loesbanluechai D, Kotanan N, de Cozar C, Kochakarn T, Ansbro MR, Chotivanich K, White NJ, Wilairat P, Lee MCS, Gamo FJ, Sanz LM, Chookajorn T, Kümpornsin K. Loesbanluechai D, et al. Int J Parasitol Drugs Drug Resist. 2019 Apr;9:16-22. doi: 10.1016/j.ijpddr.2018.11.004. Epub 2018 Dec 1. Int J Parasitol Drugs Drug Resist. 2019. PMID: 30580023 Free PMC article. - Diverse target gene modifications in Plasmodium falciparum using Bxb1 integrase and an intronic attB.
Balabaskaran-Nina P, Desai SA. Balabaskaran-Nina P, et al. Parasit Vectors. 2018 Oct 17;11(1):548. doi: 10.1186/s13071-018-3129-5. Parasit Vectors. 2018. PMID: 30333047 Free PMC article. Review. - How can we identify parasite genes that underlie antimalarial drug resistance?
Anderson T, Nkhoma S, Ecker A, Fidock D. Anderson T, et al. Pharmacogenomics. 2011 Jan;12(1):59-85. doi: 10.2217/pgs.10.165. Pharmacogenomics. 2011. PMID: 21174623 Free PMC article. Review.
References
- World Health Organization. Wkly Epidemiol Rec. 1997;72:269. - PubMed
- Dame J B, Arnot D E, Bourke P F, Chakrabarti D, Christodoulou Z, Coppel R L, Cowman A F, Craig A G, Fischer K, Foster J, et al. Mol Biochem Parasitol. 1996;79:1–12. - PubMed
- Gardner M J, Tettelin H, Carucci D J, Cummings L M, Aravind L, Koonin E V, Shallom S, Mason T, Yu K, Fujii C, et al. Science. 1998;282:1126–1132. - PubMed
- Van Dijk M R, Waters A P, Janse C J. Science. 1995;268:1358–1362. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources