Local presentation of substrate molecules directs axon specification by cultured hippocampal neurons - PubMed (original) (raw)
Local presentation of substrate molecules directs axon specification by cultured hippocampal neurons
T Esch et al. J Neurosci. 1999.
Abstract
Axon specification is a crucial, early step in neuronal development, but little is known about how this event is controlled in vivo. To test the hypothesis that local presentation of growth-promoting molecules can direct axon specification, we cultured hippocampal neurons on substrates patterned with stripes of poly-L-lysine and either laminin (LN) or the neuron-glia cell adhesion molecule (NgCAM). Although undifferentiated neurites contacted both substrates equally, axons formed preferentially on LN or NgCAM. Time-lapse studies revealed that changes in the growth pattern of a cell indicative of axon specification began almost immediately after the growth cone of one of the neurites of the cell contacted LN or NgCAM. When cells were plated on alternating stripes of LN and NgCAM, cells with their somata on LN usually formed axons on NgCAM, whereas those with somata on NgCAM preferentially formed axons on LN. This suggests that the change from one axon-promoting substrate to another also provides a signal sufficient to specify the axon. These results demonstrate that contact with preferred substrate molecules can govern which neurite becomes the axon and thus direct the development of neuronal polarity.
Figures
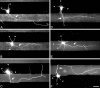
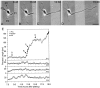
Fig. 1.
When hippocampal neurons are grown on striped substrates, axons form predominantly on one substrate. Neurons were cultured on substrates patterned with alternating stripes of P
l
L and LN (A–C) or P
l
L and NgCAM (D–F). When they were examined after 24 hr in culture, minor process growth cones (arrowheads) were positioned on both substrates, but axons (arrows) almost always formed on LN or NgCAM. Neurons were immunolabeled for tubulin, and the patterns were revealed by immunostaining for either LN or NgCAM so that P
l
L appears dark and LN or NgCAM appears_light_. Fluorescent images of neurons and stripes were superimposed. Scale bar, 25 μm.
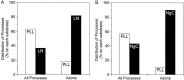
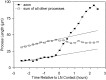
Fig. 2.
Quantification of the effects of LN and NgCAM on axon formation. The percentage of all processes and of axons that formed on each substrate was determined after 24 hr in culture.A, Distribution of processes on P
l
L/LN stripes. B, Distribution of processes on P
l
L/NgCAM stripes. If axons formed equally well on both substrates, the percentage of all processes and the percentage of axons on each substrate would be equal. Instead, most of the axons formed from a process in which the growth cone contacted LN (χ2 = 210; p < 0.001; 264 cells counted) or NgCAM (NgC) (χ2 = 64;p < 0.001; 156 cells counted). Because the cells were examined only after axons had formed, we estimated the positions of growth cones at the time when the axon was specified (see Materials and Methods). Shown are combined data from two to three experiments.
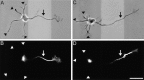
Fig. 3.
Neurons that develop on striped substrates appropriately polarize marker proteins. Cells were fixed ∼24 hr after plating and were double-immunolabeled for tau-1 and LN.A, C, Combined phase/fluorescence images showing cells positioned on stripes (LN is light).B, D, Tau-1 immunofluorescence. Tau-1 labeling is concentrated in the nascent axon (arrows) and absent from the minor processes (arrowheads) even in axons as short as 55 μm (A, B).
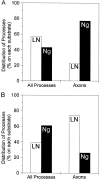
Fig. 4.
Axon specification on stripes of LN/NgCAM depends on soma position. The percentage of all processes and of axons that formed on each substrate was determined after 24 hr in culture.A, Distribution of processes for cells with somata on LN. Most axons formed from a process for which the growth cone contacted NgCAM (Ng) (χ2 = 19.28;p < 0.001; 60 cells counted). B, Distribution of cells with somata on NgCAM. Most of the axons formed from a process whose growth cone contacted LN (χ2 = 58.66; p < 0.001; 159 cells counted). Because the cells were examined only after axons had formed, we estimated the positions of growth cones at the time when the axon was specified (see Materials and Methods). Shown are combined data from six experiments.
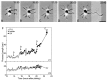
Fig. 5.
Time-lapse images showing axon formation after contact with LN. A–D, Phase micrographs photographically lightened in regions corresponding to the LN stripes.E, Length of processes over time. All minor process growth cones were on P
l
L at the beginning of the recording (A, E). Immediately after the growth cone of one process (P1) crossed onto LN (B), the process grew rapidly across the stripe (C, E). On reaching the far side of LN, the growth of P1 slowed (E), but ultimately it grew across the P
l
L stripe and acquired a length characteristic of axons (D). In E, the_letters_ correspond to the frames depicted in_A–D_; open circles represent growth cone contact with P
l
L, black circles represent growth cone contact with LN, and _black hourglasses_represent growth cone contact with a border between two substrates. Scale bar, 25 μm.
Fig. 6.
When a process contacts LN, its growth rapidly increases, whereas the growth of the remaining processes decreases. This plot compares the length of the contacting process (the axon,filled squares) with the total length of the remaining minor processes (open squares). Before contact with LN all processes exhibit the same pattern of growth. Once a single growth cone crosses onto a LN stripe, the overall behavior of the neuron changes and growth is directed primarily to the axon. Measurements from different cells (n = 9) were aligned to the time point when the process first contacted LN (time 0). Regression lines (solid lines) show the predicted trajectory if growth had continued at the same rate as before contact with LN.
Fig. 7.
When two or more processes contact LN at approximately the same time, axon formation is delayed.A–E, Phase micrographs photographically lightened in regions corresponding to the LN stripes. F, Length of the two processes that contacted LN. Initially, all processes were on P
l
L (A). Processes grew and retracted for 3–4 hr (F) before two processes (P1, P2) contacted LN within a 20 min period (B, F). Both processes retracted until their growth cones were again on P
l
L (C). Over the next 4 hr both processes grew and retracted but did not extend far onto LN (D, F). Eventually P2 developed into the axon (E). In_F_, the letters correspond to the frames depicted in A–E; open circles represent growth cone contact with P
l
L, _black circles_represent growth cone contact with LN, and black hourglasses represent growth cone contact with a border between two substrates. Scale bar, 25 μm.
Similar articles
- PIP3 is involved in neuronal polarization and axon formation.
Ménager C, Arimura N, Fukata Y, Kaibuchi K. Ménager C, et al. J Neurochem. 2004 Apr;89(1):109-18. doi: 10.1046/j.1471-4159.2004.02302.x. J Neurochem. 2004. PMID: 15030394 - Tenascin and extracellular matrix glycoproteins: from promotion to polarization of neurite growth in vitro.
Lochter A, Schachner M. Lochter A, et al. J Neurosci. 1993 Sep;13(9):3986-4000. doi: 10.1523/JNEUROSCI.13-09-03986.1993. J Neurosci. 1993. PMID: 7690068 Free PMC article. - Uncovering multiple axonal targeting pathways in hippocampal neurons.
Wisco D, Anderson ED, Chang MC, Norden C, Boiko T, Fölsch H, Winckler B. Wisco D, et al. J Cell Biol. 2003 Sep 29;162(7):1317-28. doi: 10.1083/jcb.200307069. J Cell Biol. 2003. PMID: 14517209 Free PMC article. - Changes in membrane trafficking and actin dynamics during axon formation in cultured hippocampal neurons.
Bradke F, Dotti CG. Bradke F, et al. Microsc Res Tech. 2000 Jan 1;48(1):3-11. doi: 10.1002/(SICI)1097-0029(20000101)48:1<3::AID-JEMT2>3.0.CO;2-O. Microsc Res Tech. 2000. PMID: 10620780 Review. - Axon specification in hippocampal neurons.
Fukata Y, Kimura T, Kaibuchi K. Fukata Y, et al. Neurosci Res. 2002 Aug;43(4):305-15. doi: 10.1016/s0168-0102(02)00062-7. Neurosci Res. 2002. PMID: 12135774 Review.
Cited by
- Microtubules are not required to generate a nascent axon in embryonic spinal neurons in vivo.
Moore RE, Pop S, Alleyne C, Clarke JDW. Moore RE, et al. EMBO Rep. 2022 Nov 7;23(11):e52493. doi: 10.15252/embr.202152493. Epub 2022 Oct 4. EMBO Rep. 2022. PMID: 36194673 Free PMC article. - Activated c-Jun N-terminal kinase is required for axon formation.
Oliva AA Jr, Atkins CM, Copenagle L, Banker GA. Oliva AA Jr, et al. J Neurosci. 2006 Sep 13;26(37):9462-70. doi: 10.1523/JNEUROSCI.2625-06.2006. J Neurosci. 2006. PMID: 16971530 Free PMC article. - Substrate topography determines neuronal polarization and growth in vitro.
Micholt L, Gärtner A, Prodanov D, Braeken D, Dotti CG, Bartic C. Micholt L, et al. PLoS One. 2013 Jun 13;8(6):e66170. doi: 10.1371/journal.pone.0066170. Print 2013. PLoS One. 2013. PMID: 23785482 Free PMC article. - Shootin1: A protein involved in the organization of an asymmetric signal for neuronal polarization.
Toriyama M, Shimada T, Kim KB, Mitsuba M, Nomura E, Katsuta K, Sakumura Y, Roepstorff P, Inagaki N. Toriyama M, et al. J Cell Biol. 2006 Oct 9;175(1):147-57. doi: 10.1083/jcb.200604160. J Cell Biol. 2006. PMID: 17030985 Free PMC article. - Gradients of substrate-bound laminin orient axonal specification of neurons.
Dertinger SK, Jiang X, Li Z, Murthy VN, Whitesides GM. Dertinger SK, et al. Proc Natl Acad Sci U S A. 2002 Oct 1;99(20):12542-7. doi: 10.1073/pnas.192457199. Epub 2002 Sep 17. Proc Natl Acad Sci U S A. 2002. PMID: 12237407 Free PMC article.
References
- Bixby JL. Protein kinase C is involved in laminin stimulation of neurite outgrowth. Neuron. 1989;3:287–297. - PubMed
- Bottenstein JE. Growth and differentiation of neural cells in defined media. In: Bottenstein JE, Sato G, editors. Cell culture in the neurosciences. Plenum; New York: 1985. pp. 3–44.
- Bradke F, Dotti CG. The role of local actin instability in axon formation. Science. 1999;283:1931–1934. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources