Glutamate release through volume-activated channels during spreading depression - PubMed (original) (raw)
Glutamate release through volume-activated channels during spreading depression
T A Basarsky et al. J Neurosci. 1999.
Abstract
Volume-sensitive organic anion channels (VSOACs) in astrocytes are activated by cell swelling and are permeable to organic anions, such as glutamate and taurine. We have examined the release of glutamate through VSOACs during the propagation of spreading depression (SD). SD was induced by bath application of ouabain in hippocampal brain slices and was monitored by imaging intrinsic optical signals, a technique that provides a measure of cellular swelling. The onset of SD was associated with increased light transmittance, confirming previous studies that cellular swelling occurs during SD. NMDA receptor antagonists, either noncompetitive (MK-801, 10-50 microM) or competitive (CGS-17355, 100 microM), reduced the rate of propagation of SD, indicating that glutamate release contributes to SD onset. SD still occurred in zero Ca(2+)-EGTA (0-Ca(2+)-EGTA) solution, a manipulation that depresses synaptic transmission. HPLC measurements indicated that, even in this solution, there was significant glutamate release. Two lines of experiments indicated that glutamate was released through VSOACs during SD. First, 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB), a blocker of VSOACs, depressed the rate of propagation of SD in a manner similar to NMDA antagonists. Second, NPPB inhibited the release of glutamate during SD in 0-Ca(2+)-EGTA external solution. These results indicate that cellular swelling during SD causes the activation of VSOACs and the release of glutamate by permeation through this channel. Cellular swelling is a result of neuronal activity and is observed during excitotoxicity. Therefore, glutamate release from VSOAC activation could occur under conditions of cell swelling and contribute to excitotoxic damage.
Figures
Fig. 1.
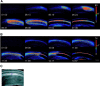
Time course of ouabain-induced spreading depression in hippocampal slices. A, Spreading depression in control conditions. SD rapidly initiated in CA1 and propagated throughout the entire CA1 region as a wave of increased light transmittance followed by a rapid decrease. B, Spreading depression in the presence of 50 μ
m
MK-801, after preincubation for 20 min in MK-801. The waveform of SD was different in that the initial increase in light transmittance was slow, but this was still followed by a rapidly propagating wave of decreased transmittance. C, Bright-field image of the slice used in A. The purple dot denotes a typical region used for measurements, and the CA1 region has been shown in_A_ and B. Ouabain (100 μ
m
) was added at 1 minute 30 seconds. All times are given in minutes:seconds. Scale bar, 100 μm.
Fig. 2.
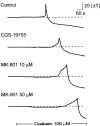
Time course of spreading depression in the presence of NMDA receptor antagonists. Each trace_represents the change in transmittance in a single region of interest (as denoted in Fig. 1) recorded in separate brain slices. The_top trace is a profile of spreading depression in a control slice. The bottom three traces are profiles of spreading depression in separate brain slices incubated in the indicated NMDA receptor antagonist. Both the competitive antagonist (CGS-19755) and the noncompetitive antagonist (MK-801) reduced the onset of SD, and the effects of MK-801 were dose-dependent.Solid bar represents the time of application of ouabain (100 μ
m
).
Fig. 3.
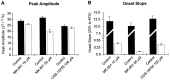
Quantification of the effects of NMDA receptor antagonists on spreading depression. A, The amplitude of the peak response was only reduced significantly by 50 μ
m
MK-801 (p < 0.01) but was not significantly affected by CGS-19755 (100 μ
m
) or MK-801 (10 μ
m
) (p > 0.05).B, The onset slope was reduced by both the competitive (CGS-19755) and noncompetitive (MK-801) NMDA receptor antagonists. Note that the scale in B is split to enable the display of both control and antagonist measurements.
Fig. 4.
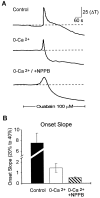
NPPB, a VSOAC antagonist, inhibited the onset of spreading depression. A, Representative_traces_ of the light transmittance changes in the presence and absence of external calcium and in the presence of the VSOAC antagonist NPPB (100 μ
m
). B, Summary data for the effects of 0-Ca2+ and NPPB on the onset slope of spreading depression. In the absence of calcium, NPPB significantly inhibited the onset slope. _Solid bar_denotes the time of application of ouabain (100 μ
m
).
Fig. 5.
Inhibition of VSOACs inhibited the calcium-independent release of glutamate. All data were collected from brain slices incubated in 0-Ca2+–EGTA solution for at least 20 min before the start of the experiment. A, Representative HPLC measurements of glutamate release in the superfusate before and during SD in two separate brain slices. The initial onset of SD was measured using intrinsic optical signals and is taken as t = 0. SD propagated throughout the brain slice during the collection of superfusate and induced the release of glutamate, even in the 0-Ca2+–EGTA solution. Preincubation with NPPB (100 μ
m
) reduced the release of glutamate during spreading depression. B, Effect of NPPB on glutamate, GABA, and glutamine release during spreading depression. Each point represents the increase in the indicated amino acid 7 min after the initiation of spreading depression from at least n = 5 separate brain slices. All numbers are expressed normalized to the initial levels before spreading depression. NPPB significantly depressed the release of only glutamate during SD (*p < 0.01) and had no effect on the release of either GABA or glutamine.
Similar articles
- NMDA-receptor blockers but not NBQX, an AMPA-receptor antagonist, inhibit spreading depression in the rat brain.
Nellgård B, Wieloch T. Nellgård B, et al. Acta Physiol Scand. 1992 Dec;146(4):497-503. doi: 10.1111/j.1748-1716.1992.tb09451.x. Acta Physiol Scand. 1992. PMID: 1283483 - ATP stimulates calcium-dependent glutamate release from cultured astrocytes.
Jeremic A, Jeftinija K, Stevanovic J, Glavaski A, Jeftinija S. Jeremic A, et al. J Neurochem. 2001 Apr;77(2):664-75. doi: 10.1046/j.1471-4159.2001.00272.x. J Neurochem. 2001. PMID: 11299329 - Regenerative glutamate release by presynaptic NMDA receptors contributes to spreading depression.
Zhou N, Rungta RL, Malik A, Han H, Wu DC, MacVicar BA. Zhou N, et al. J Cereb Blood Flow Metab. 2013 Oct;33(10):1582-94. doi: 10.1038/jcbfm.2013.113. Epub 2013 Jul 3. J Cereb Blood Flow Metab. 2013. PMID: 23820646 Free PMC article. - Implication of glutamate in the expression of inducible nitric oxide synthase after oxygen and glucose deprivation in rat forebrain slices.
Cárdenas A, Moro MA, Hurtado O, Leza JC, Lorenzo P, Castrillo A, Bodelón OG, Boscá L, Lizasoain I. Cárdenas A, et al. J Neurochem. 2000 May;74(5):2041-8. doi: 10.1046/j.1471-4159.2000.0742041.x. J Neurochem. 2000. PMID: 10800947
Cited by
- Astrocytic Glutamatergic Transmission and Its Implications in Neurodegenerative Disorders.
Satarker S, Bojja SL, Gurram PC, Mudgal J, Arora D, Nampoothiri M. Satarker S, et al. Cells. 2022 Mar 28;11(7):1139. doi: 10.3390/cells11071139. Cells. 2022. PMID: 35406702 Free PMC article. Review. - Striatal spreading depolarization: Possible implication in levodopa-induced dyskinetic-like behavior.
de Iure A, Napolitano F, Beck G, Quiroga Varela A, Durante V, Sciaccaluga M, Mazzocchetti P, Megaro A, Tantucci M, Cardinale A, Punzo D, Mancini A, Costa C, Ghiglieri V, Tozzi A, Picconi B, Papa SM, Usiello A, Calabresi P. de Iure A, et al. Mov Disord. 2019 Jun;34(6):832-844. doi: 10.1002/mds.27632. Epub 2019 Feb 13. Mov Disord. 2019. PMID: 30759320 Free PMC article. - Zn2+ influx is critical for some forms of spreading depression in brain slices.
Dietz RM, Weiss JH, Shuttleworth CW. Dietz RM, et al. J Neurosci. 2008 Aug 6;28(32):8014-24. doi: 10.1523/JNEUROSCI.0765-08.2008. J Neurosci. 2008. PMID: 18685026 Free PMC article. - Volume-regulated anion channel--a frenemy within the brain.
Mongin AA. Mongin AA. Pflugers Arch. 2016 Mar;468(3):421-41. doi: 10.1007/s00424-015-1765-6. Epub 2015 Dec 1. Pflugers Arch. 2016. PMID: 26620797 Free PMC article. Review. - Neuroprotection by Delta9-tetrahydrocannabinol, the main active compound in marijuana, against ouabain-induced in vivo excitotoxicity.
van der Stelt M, Veldhuis WB, Bär PR, Veldink GA, Vliegenthart JF, Nicolay K. van der Stelt M, et al. J Neurosci. 2001 Sep 1;21(17):6475-9. doi: 10.1523/JNEUROSCI.21-17-06475.2001. J Neurosci. 2001. PMID: 11517236 Free PMC article.
References
- Andrew RD, MacVicar BA. Imaging cell volume changes and neuronal excitation in the hippocampal slice. Neuroscience. 1994;62:371–383. - PubMed
- Duan D, Winter C, Cowley S, Hume JR, Horowitz B. Molecular identification of a volume-regulated chloride channel. Nature. 1997;390:417–421. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous