Isolectin B(4)-positive and -negative nociceptors are functionally distinct - PubMed (original) (raw)
Isolectin B(4)-positive and -negative nociceptors are functionally distinct
C L Stucky et al. J Neurosci. 1999.
Abstract
Small-diameter sensory neurons that are primarily nociceptors can be divided neurochemically into two populations: isolectin B(4) (IB(4))-positive nonpeptidergic neurons, and IB(4)-negative peptidergic neurons. It has been shown that IB(4)-positive neurons depend on glial-derived neurotrophic factor (GDNF), whereas IB(4)-negative neurons depend on NGF for survival during postnatal development (Molliver et al., 1997). Furthermore, these two populations of nociceptors terminate in distinct regions of the superficial spinal cord. To date, however, no evidence exists that indicates whether these two groups of nociceptors have distinct functional roles in the process of nociception (Snider and McMahon, 1998). To search for functional differences, we performed whole-cell voltage and current-clamp recordings on acutely isolated adult mouse dorsal root ganglion neurons that were labeled with fluorescent IB(4). We found that IB(4)-positive neurons have longer-duration action potentials, higher densities of TTX-resistant sodium currents, and smaller noxious heat-activated currents than IB(4)-negative neurons. Furthermore, we show that NGF, but not GDNF, directly increases the number of neurons that respond to noxious heat. The different electrophysiological properties expressed by IB(4)-positive and -negative small neurons, including their different heat sensitivities, indicates that they may relay distinct aspects of noxious stimuli both acutely and after injury in vivo.
Figures
Fig. 1.
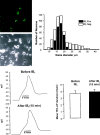
A, Left, Fluorescent and phase-contrast images of field of sensory neurons fixed after labeling with IB4 -FITC. Right, Distribution of IB4-positive and -negative sensory neurons by soma diameter. Dorsal root ganglion neurons from all spinal levels were isolated, placed in culture, and then fixed and stained 24 hr later (n = 4 mice, 530 neurons). B,Left, Profile of a somatic action potential (AP) before (top) and after (bottom) addition of IB4-FITC. This neuron was IB4 positive. Note that binding of IB4 did not alter the shape or duration of the AP. Right, In six IB4-positive neurons, IB4 binding did not alter the duration of the somatic AP.
Fig. 2.
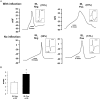
A, Examples of APs in IB4-positive and -negative neurons with (top) and without (bottom) inflections on the falling phase. Insets show the first derivative of each spike and illustrate that the APs in the top have an inflection, whereas those in bottom do not. Note that IB4-positive neurons have longer duration APs than IB4-negative neurons. B, Mean duration of AP measured at 75% of spike amplitude. The _asterisk_indicates that the duration of APs in IB4-positive neurons is significantly longer than in IB4-negative neurons (p < 0.05; t test).
Fig. 3.
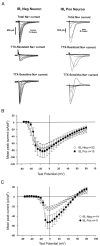
A, Examples of whole-cell voltage-clamp recordings under conditions selective for Na+ currents in an IB4-negative and an IB4-positive neuron. TTX-resistant Na+current was recorded in the presence of 1 μ
m
TTX, and TTX-sensitive current was acquired by electronically subtracting the TTX-resistant current from the Total Na+current. The overshoot at one test-pulse in the calculated TTX-sensitive component of the IB4-positive neuron was caused by variability in the voltage clamp and electronically subtracting the TTX-resistant current from the total current.B, Mean TTX-sensitive Na+ current density for all neurons at different test potentials. Peak TTX-sensitive currents were divided by total cell capacitance to give the current density. C, Mean TTX-resistant Na+ current density for all neurons. TTX-resistant currents were recorded in the presence of 1 μ
m
TTX, and peak currents were corrected for cell capacitance.
Fig. 4.
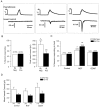
A, Examples of whole-cell voltage-clamp recordings from three different small-diameter neurons and their responses to a ramp of heated buffer applied to cell membrane (24–49°C in 10 ± 1 sec). Neuron that did not respond to heat (left) was IB4 negative, neuron with small inward current (middle) was IB4 positive, and neuron with large inward current (right) was IB4 negative. B, Left, Percentage of IB4-positive and -negative neurons that responded to a noxious heat stimulus. Right, Magnitude of heat-evoked currents. The asterisk indicates that currents in IB4-negative neurons were significantly larger than those in IB4-positive neurons (p < 0.05; χ2 test).C, Cultures of neurons were treated with no growth factor (Control), NGF (100 ng/ml), or GDNF (50 ng/ml) for 24 hr and then tested for responses to noxious heat. Values in parentheses_indicate the number of responding cells per number of cells tested for each group. Single asterisk indicates that percentage of responding cells in NGF-treated IB4-negative neurons is significantly different from that of IB4-negative control neurons. Double asterisk indicates that percentage of responding cells in NGF-treated IB4-positive neurons is significantly different from that of IB4-positive control neurons. D, Mean inward currents evoked in neurons treated with no growth factor, NGF, or GDNF for 24 hr. Values in_parentheses indicate the number of responding cells in each group.
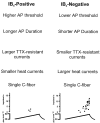
Fig. 5.
Putative roles of IB4-positive and -negative neurons in nociceptive function.
Similar articles
- GFR alpha2/neurturin signalling regulates noxious heat transduction in isolectin B4-binding mouse sensory neurons.
Stucky CL, Rossi J, Airaksinen MS, Lewin GR. Stucky CL, et al. J Physiol. 2002 Nov 15;545(1):43-50. doi: 10.1113/jphysiol.2002.027656. J Physiol. 2002. PMID: 12433948 Free PMC article. - Differential response properties of IB(4)-positive and -negative unmyelinated sensory neurons to protons and capsaicin.
Dirajlal S, Pauers LE, Stucky CL. Dirajlal S, et al. J Neurophysiol. 2003 Jan;89(1):513-24. doi: 10.1152/jn.00371.2002. J Neurophysiol. 2003. PMID: 12522198 - Tetrodotoxin-sensitive and -resistant Na+ channel currents in subsets of small sensory neurons of rats.
Wu ZZ, Pan HL. Wu ZZ, et al. Brain Res. 2004 Dec 17;1029(2):251-8. doi: 10.1016/j.brainres.2004.09.051. Brain Res. 2004. PMID: 15542080 - Transgenic Mouse Models for the Tracing of “Pain” Pathways.
Basbaum AI, Bráz JM. Basbaum AI, et al. In: Kruger L, Light AR, editors. Translational Pain Research: From Mouse to Man. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 7. In: Kruger L, Light AR, editors. Translational Pain Research: From Mouse to Man. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 7. PMID: 21882471 Free Books & Documents. Review. - Painful channels in sensory neurons.
Lee Y, Lee CH, Oh U. Lee Y, et al. Mol Cells. 2005 Dec 31;20(3):315-24. Mol Cells. 2005. PMID: 16404144 Review.
Cited by
- A modulator of the low-voltage-activated T-type calcium channel that reverses HIV glycoprotein 120-, paclitaxel-, and spinal nerve ligation-induced peripheral neuropathies.
Cai S, Tuohy P, Ma C, Kitamura N, Gomez K, Zhou Y, Ran D, Bellampalli SS, Yu J, Luo S, Dorame A, Yen Ngan Pham N, Molnar G, Streicher JM, Patek M, Perez-Miller S, Moutal A, Wang J, Khanna R. Cai S, et al. Pain. 2020 Nov;161(11):2551-2570. doi: 10.1097/j.pain.0000000000001955. Pain. 2020. PMID: 32541387 Free PMC article. - Molecular and functional expression of cation-chloride cotransporters in dorsal root ganglion neurons during postnatal maturation.
Mao S, Garzon-Muvdi T, Di Fulvio M, Chen Y, Delpire E, Alvarez FJ, Alvarez-Leefmans FJ. Mao S, et al. J Neurophysiol. 2012 Aug 1;108(3):834-52. doi: 10.1152/jn.00970.2011. Epub 2012 Mar 28. J Neurophysiol. 2012. PMID: 22457464 Free PMC article. - Glial cell line-derived neurotrophic factor is a survival factor for isolectin B4-positive, but not vanilloid receptor 1-positive, neurons in the mouse.
Zwick M, Davis BM, Woodbury CJ, Burkett JN, Koerber HR, Simpson JF, Albers KM. Zwick M, et al. J Neurosci. 2002 May 15;22(10):4057-65. doi: 10.1523/JNEUROSCI.22-10-04057.2002. J Neurosci. 2002. PMID: 12019325 Free PMC article. - Chemosensory properties of murine nasal and cutaneous trigeminal neurons identified by viral tracing.
Damann N, Rothermel M, Klupp BG, Mettenleiter TC, Hatt H, Wetzel CH. Damann N, et al. BMC Neurosci. 2006 Jun 8;7:46. doi: 10.1186/1471-2202-7-46. BMC Neurosci. 2006. PMID: 16762059 Free PMC article. - Transgenic overexpression of galanin in the dorsal root ganglia modulates pain-related behavior.
Holmes FE, Bacon A, Pope RJ, Vanderplank PA, Kerr NC, Sukumaran M, Pachnis V, Wynick D. Holmes FE, et al. Proc Natl Acad Sci U S A. 2003 May 13;100(10):6180-5. doi: 10.1073/pnas.0937087100. Epub 2003 Apr 29. Proc Natl Acad Sci U S A. 2003. PMID: 12721371 Free PMC article.
References
- Aguayo LG, White G. Effects of nerve growth factor on TTX- and capsaicin-sensitivity in adult rat sensory neurons. Brain Res. 1992;570:61–67. - PubMed
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. - PubMed
- Belyantseva IA, Lewin GR. Stability and plasticity of primary afferent projections following nerve regeneration and central degeneration. Eur J Neurosci. 1999;11:457–469. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources