Proline-rich synapse-associated protein-1/cortactin binding protein 1 (ProSAP1/CortBP1) is a PDZ-domain protein highly enriched in the postsynaptic density - PubMed (original) (raw)
Proline-rich synapse-associated protein-1/cortactin binding protein 1 (ProSAP1/CortBP1) is a PDZ-domain protein highly enriched in the postsynaptic density
T M Boeckers et al. J Neurosci. 1999.
Abstract
The postsynaptic density (PSD) is crucially involved in the structural and functional organization of the postsynaptic neurotransmitter reception apparatus. Using antisera against rat brain synaptic junctional protein preparations, we isolated cDNAs coding for proline-rich synapse-associated protein-1 (ProSAP1), a PDZ-domain protein. This protein was found to be identical to the recently described cortactin-binding protein-1 (CortBP1). Homology screening identified a related protein, ProSAP2. Specific antisera raised against a C-terminal fusion construct and a central part of ProSAP1 detect a cluster of immunoreactive bands of 180 kDa in the particulate fraction of rat brain homogenates that copurify with the PSD fraction. Transcripts and immunoreactivity are widely distributed in the brain and are upregulated during the period of synapse formation in the brain. In addition, two short N-terminal insertions are detected; they are differentially regulated during brain development. Confocal microscopy of hippocampal neurons showed that ProSAP1 is predominantly localized in synapses, and immunoelectron microscopy in situ revealed a strong association with PSDs of hippocampal excitatory synapses. The accumulation of ProSAP1 at synaptic structures was analyzed in the developing cerebral cortex. During early postnatal development, strong immunoreactivity is detectable in neurites and somata, whereas from postnatal day 10 (P10) onward a punctate staining is observed. At the ultrastructural level, the immunoreactivity accumulates at developing PSDs starting from P8. Both interaction with the actin-binding protein cortactin and early appearance at postsynaptic sites suggest that ProSAP1/CortBP1 may be involved in the assembly of the PSD during neuronal differentiation.
Figures
Fig. 1.
Primary structure of ProSAP1. A, Physical map of the rat ProSAP1 cDNA. The protein coding region is_boxed_. The PDZ and SAM domain are indicated in_light_ and dark gray, respectively. The SH3 interaction module (ppI) is_hatched_; proline-rich elements (5) are indicated by_thin lines_. The positions of two alternatively processed inserts (A and B) are marked. In the 3′-untranslated region a polyA-tail (beginning at nucleotide 7688) is found (compare accession no. AJ131899). B, Alignment of the amino acid sequences of rat ProSAP1 (as deduced from the cDNA, top sequence) and synamon (EMBL/GenBank accession no. AF102855, bottom sequence). Regions of high homology are shaded in_gray_; PDZ domain is lined in black; insertions A and B in ProSAP1 are indicated by double-headed arrows; the SH3-binding motif and proline-rich elements are underlined with_bold_ and broken lines, respectively; the N-terminal SAM domain (displayed as inverted characters) is not conserved between the two proteins. The ankyrin repeats of synamon are underlined, and the SH3 domain of synamon is_boxed_ in broken lines. C, ProSAP1, ProSAP2, and synamon PDZ domains define a new subfamily. Shown is alignment of the PDZ domains of rat ProSAP1, rat synamon, rat and human ProSAP2 (accession nos. AJ131899, AF102855, AJ133120, AC000050, and AC000036) with examples of a more distantly related PDZ domain of synaptic membrane-associated guanylate kinases of SAP90/PSD-95 (Kistner et al., 1993), SAP97 (Müller et al., 1995), SAP102 (Müller et al., 1996), Chapsyn-110/PSD-93 (Kim et al., 1996; Brenman et al., 1996a,b), and DLG1 (Woods and Bryant, 1989).
Fig. 2.
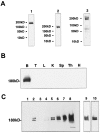
Tissue distribution and subcellular co-partitioning of ProSAP1 in rat brain A antisera generated against ProSAP1 (1, polyclonal rabbit antiserum, C-Term aa 826–1259; 2, polyclonal guinea pig antibody, C-Term aa 826–1259; 3, polyclonal rabbit antiserum, central part, aa 355–509) detect a major protein band at 180 kDa and two weaker bands at ∼200 and 220 kDa in rat brain protein preparations.B, ProSAP1 immunoreactivity is only detectable on immunoblots of rat brain protein preparations and not found in testis (T), liver (L), kidney (K), spleen (Sp), thymus (Th), or heart muscle (H). Protein extracts were obtained from 8-week-old rats. Western blots were loaded with 50 μg per slot of detergent-insoluble cytoskeletal protein. C, ProSAP1 is highly enriched in synaptic junctional protein preparation. Synaptic proteins were prepared according to Carlin et al. (1980). Western blots (15 μg protein per lane) of the soluble protein fraction (lane 1), the crude membrane fraction P2 (lane 2), the myelin fraction (lane 3), the light membranes fraction (lane 4), the synaptosomal fraction (lane 5), detergent extracted synaptosomes (lane 6) [i.e., One Triton, Kennedy (1997)], postsynaptic density fraction obtained from the detergent extracted fraction in a second gradient (lane 7), and the twice Triton X-100-extracted PSD fraction (lane 8) [Two Triton, Kennedy (1997)] were probed with the rabbit anti-ProSAP1 antibody using a chemiluminescent detection system. Lane 9 shows the twice Triton X-100-extracted PSD fraction (lane 8) with a shorter exposition time; lane 10 displays a Western blot of the twice Triton X-100-extracted PSD fraction with less protein loaded (3 μg). Note that the distribution of other synaptic proteins, including the presynaptic cytomatrix proteins Bassoon and Piccolo, the PSD proteins SAP102, and the synaptic vesicle protein synaptophysin in the presented subcellular fractionation experiment, has been assessed previously (tom Dieck et al., 1998).
Fig. 3.
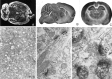
Distribution of ProSAP1 in the adult rat brain.A, Distribution of mRNA transcripts. In situ hybridization to a horizontal section from brain with the35S-labeled ProSAP1 antisense oligonucleotide shows the overall expression of the transcript in the adult brain. Intense labeling is observed in cerebral cortex, cerebellum, hippocampus, and olfactory bulb, whereas putamen, thalamic nuclei, and brainstem show a moderate staining. Magnification: 2.5×. B,C, Overview of spatial distribution of ProSAP1 protein in rat brain by immunohistochemistry with ProSAP1 antisera. Sagittal (B) and frontal (C) sections are shown. Strong ProSAP1 immunoreactivity is detected in cerebral cortex, hippocampus, and the molecular layer of the cerebellum. Furthermore, the thalamic nuclei, the putamen, and to a much lesser extent the hypothalamus are labeled. Further enlargement of the hippocampal CA2/CA3 region (D) illustrates that cell nuclei and cell bodies are free of staining, whereas in the neuropil a punctate staining (arrows) can be observed. Magnifications: B, C, 2.5×; D, 450×. Electron micrographs (E, F) were taken from hippocampal CA3 sections. Silver enhancement of the DAB reaction product results in the punctate appearance of the immunoreactivity. Note that labeling is relatively weak in dendrites (d), enhanced toward dendritic spines (sp), and very strong at PSDs (arrowheads, arrows). Axon terminals (at) are essentially unlabeled. Magnification: F, 45,000×; G, 85,000×.
Fig. 4.
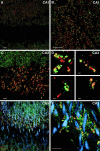
Distribution of ProSAP1 in hippocampal neurons as revealed by double- and triple-immunofluorescence labeling.A_–_D, Double immunofluorescence of hippocampal neurons in the CA1 and CA3 region with the rabbit antibody directed against ProSAP1 (CY3, green) and a monoclonal antibody directed against the presynaptic protein Bassoon (A, B, D, CY4, red) or synaptophysin (C, CY4, red). Note that the antigens are largely co-distributed at hippocampal synapses. At higher magnifications of mossy fiber terminals in the stratum lucidum of the CA3 region (C, D), the close apposition of ProSAP1 with both presynaptic marker proteins can be seen. The staining with the Bassoon antibody especially illustrates the close spatial relationship of the two proteins because Bassoon is mainly restricted to the active zone of the presynapse (tom Dieck et al., 1998; Richter et al., 1999). Confocal images of triple immunofluorescence ProSAP1 (green), Bassoon (red), and MAP2 (blue) (E) as well as ProSAP1 (green), synapsin (red), and MAP2 (blue) (F) show the localization of synaptic structures on dendrites of hippocampal CA3 neurons. Please note that labeled shaft and spine synapses are discernible that decorate the MAP2-positive dendritic trees. Scale bars: A–C, E, F, 10 μm; D (all_insets_), 1 μm.
Fig. 5.
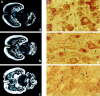
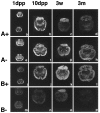
A, Distribution of the ProSAP1 transcripts and protein in the developing rat brain. In situ hybridizations of horizontal brain sections from 5 (a)-, 9 (b)-, and 18-d-old (c) rats. X-ray film images of in situ hybridizations with the ProSAP1 antisense oligonucleotide show the dense expression of the transcript in cortex, cerebellum, and hippocampus at these developmental stages. The transcript is especially upregulated during development in the thalamic nuclei and the caudate putamen. Magnification: 3.5×. B, Immunohistochemical staining of cortical neurons at P5 (a), P8 (b), and P10 (c). Note the strong labeling of cytoplasm and small outgrowing neurites at P5 (a), whereas the neuropil appears largely unstained. On P8 the cytoplasmic staining is reduced, and larger neurites (arrows) are clearly labeled (b). Two days later (P10) the staining pattern changes to a punctate labeling (arrows) in the neuropil of the developing cortex (c). Magnification: 500×.
Fig. 6.
Ultrastructural localization of ProSAP1 in the developing rat cortex. Electronmicroscopy of immunostained cortical sections at P5 (A, B) shows the primarily cytoplasmic localization of ProSAP1 in a subset of outgrowing neurites. Note the clearcut differentiation between ProSAP1-positive and -negative neurites. In A, a ProSAP1-positive neurite with putatively pathfinding lamellopodia is displayed. _B_shows the close contact between a ProSAP1-positive and -negative neurite. At P8, strong labeling can be found in the cytoplasm of growing neurites (C); synaptic contacts show strong ProSAP1 immunoreactivity in the now appearing PSDs (D, E). At P10 (F, G), differentiation of brain tissue has advanced, and ProSAP1 immunoreactivity is primarily found in spines and in particular at PSDs. Magnification: A, B, 46,000×; C, D, 70,000×; E, 90,000×.at, Axon terminal.
Fig. 7.
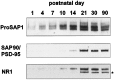
Developmental association of ProSAP1, SAP90/PSD-95, and the NR1 subunit of the NMDA receptor with cytoskeletal protein fractions. Western blot analysis of a fraction enriched for PSD elements (20 μg/lane) during postnatal development shows that moderate amounts of ProSAP1 are already detectable at P1 with a significant increase of the signal intensity between P7 and P10. Association of SAP90/PSD-95 with the protein fraction strongly increases from P14 onward. The detection of the NR1 subunit of the NMDA receptor is detectable from P1, but a strong increase in immunoreactivity is seen only between P10 and P21. The_star_ indicates the band resulting from the SAP90/PSD-95 staining. The blot has been reprobed for the detection of the NR1 subunit of the NMDA receptor.
Fig. 8.
Expression of processing variants of ProSAP1 transcripts in the brain during postnatal development. During early postnatal development, insert A (a, b, A+) shows a wide distribution throughout the brain. At later stages the hybridization signal intensity decreases and becomes mainly restricted to the cerebellum (c, d, A+). ProSAP1 transcripts without insert A show a wide expression in rat brain at all developmental stages investigated (e–h, A−). Nearly identical results were obtained with an antisense oligonucleotide designed to recognize insert B (i–l, B+). In contrast, ProSAP1 mRNA without insert B shows a very weak expression on P1 and P10; it is largely restricted to the cerebellum after 3 weeks and cannot be detected in the adult rat brain. Therefore in most brain regions the_A−/B+_ transcript seems to be regularly expressed, whereas during development, especially in the cerebellum, transcripts with insert A but without insert B can also be detected.
Similar articles
- Proline-rich synapse-associated proteins ProSAP1 and ProSAP2 interact with synaptic proteins of the SAPAP/GKAP family.
Boeckers TM, Winter C, Smalla KH, Kreutz MR, Bockmann J, Seidenbecher C, Garner CC, Gundelfinger ED. Boeckers TM, et al. Biochem Biophys Res Commun. 1999 Oct 14;264(1):247-52. doi: 10.1006/bbrc.1999.1489. Biochem Biophys Res Commun. 1999. PMID: 10527873 - Proline-rich synapse-associated protein-1/cortactin binding protein 1 (ProSAP1/CortBP1) is a PDZ-domain protein highly enriched in the postsynaptic density.
Boeckers TM, Kreutz MR, Winter C, Zuschratter W, Smalla KH, Sanmarti-Vila L, Wex H, Langnaese K, Bockmann J, Garner CC, Gundelfinger ED. Boeckers TM, et al. Ann Anat. 2001 Mar;183(2):101. doi: 10.1016/S0940-9602(01)80024-8. Ann Anat. 2001. PMID: 11325055 No abstract available. - Interaction of G-protein-coupled receptors with synaptic scaffolding proteins.
Kreienkamp HJ, Soltau M, Richter D, Böckers T. Kreienkamp HJ, et al. Biochem Soc Trans. 2002 Aug;30(4):464-8. doi: 10.1042/bst0300464. Biochem Soc Trans. 2002. PMID: 12196116 Review. - ProSAP/Shank proteins - a family of higher order organizing molecules of the postsynaptic density with an emerging role in human neurological disease.
Boeckers TM, Bockmann J, Kreutz MR, Gundelfinger ED. Boeckers TM, et al. J Neurochem. 2002 Jun;81(5):903-10. doi: 10.1046/j.1471-4159.2002.00931.x. J Neurochem. 2002. PMID: 12065602 Review.
Cited by
- Expression profiles of the autism-related SHANK proteins in the human brain.
Woelfle S, Pedro MT, Wagner J, Schön M, Boeckers TM. Woelfle S, et al. BMC Biol. 2023 Nov 13;21(1):254. doi: 10.1186/s12915-023-01712-0. BMC Biol. 2023. PMID: 37953224 Free PMC article. - Abelson interacting protein 1 (Abi-1) is essential for dendrite morphogenesis and synapse formation.
Proepper C, Johannsen S, Liebau S, Dahl J, Vaida B, Bockmann J, Kreutz MR, Gundelfinger ED, Boeckers TM. Proepper C, et al. EMBO J. 2007 Mar 7;26(5):1397-409. doi: 10.1038/sj.emboj.7601569. Epub 2007 Feb 15. EMBO J. 2007. PMID: 17304222 Free PMC article. - Sociability and motor functions in Shank1 mutant mice.
Silverman JL, Turner SM, Barkan CL, Tolu SS, Saxena R, Hung AY, Sheng M, Crawley JN. Silverman JL, et al. Brain Res. 2011 Mar 22;1380:120-37. doi: 10.1016/j.brainres.2010.09.026. Epub 2010 Sep 21. Brain Res. 2011. PMID: 20868654 Free PMC article. - The actin-driven movement and formation of acetylcholine receptor clusters.
Dai Z, Luo X, Xie H, Peng HB. Dai Z, et al. J Cell Biol. 2000 Sep 18;150(6):1321-34. doi: 10.1083/jcb.150.6.1321. J Cell Biol. 2000. PMID: 10995438 Free PMC article. - Key role of the postsynaptic density scaffold proteins Shank and Homer in the functional architecture of Ca2+ homeostasis at dendritic spines in hippocampal neurons.
Sala C, Roussignol G, Meldolesi J, Fagni L. Sala C, et al. J Neurosci. 2005 May 4;25(18):4587-92. doi: 10.1523/JNEUROSCI.4822-04.2005. J Neurosci. 2005. PMID: 15872106 Free PMC article.
References
- Abeliovich A, Chen C, Goda Y, Silva AJ, Stevens CF, Tonegawa S. Modified hippocampal long-term potentiation in PKC gamma-mutant mice. Cell 31 1993. 75:1253–1262. - PubMed
- Adam G, Matus A. Role of actin in the organisation of brain postsynaptic densities. Brain Res Mol Brain Res. 1996;31:246–250. - PubMed
- Ben-Ari Y, Aniksztejn L, Bregestovski P. Protein kinase C modulation on NMDA currents: an important link for LTP induction. Trends Neurosci. 1992;15:333–339. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials