Basolateral amygdala is involved in modulating consolidation of memory for classical fear conditioning - PubMed (original) (raw)
Basolateral amygdala is involved in modulating consolidation of memory for classical fear conditioning
A Vazdarjanova et al. J Neurosci. 1999.
Abstract
Previous findings indicate that the basolateral amygdala complex of nuclei (BLC) is involved in modulating (i.e., enhancing or impairing) memory consolidation for aversive training such as inhibitory avoidance. The present study examined whether the BLC also modulates the consolidation of memory for classical fear conditioning in which a specific context is paired with footshock. Adult male rats with bilateral cannulae targeting the BLC were allowed, first, to habituate in a Y maze that had differently shaped and textured arms. On the next day the rats were placed in one maze arm (shock arm), and they received four unsignaled footshocks. In Experiment 1, immediately after the training some rats received BLC inactivation with lidocaine (10 microgram/0.2 microliter per side), and control rats received buffered saline. In Experiment 2, rats received immediate post-training intra-BLC infusions of the muscarinic receptor agonist oxotremorine (10 ng/0.2 microliter per side) or saline. On a 24 hr retention test each rat was placed in a "safe" arm of the maze and allowed to access all maze arms. Lidocaine-treated rats had impaired memory for the classical fear conditioning when they were compared with the saline-treated controls: they spent less time freezing, entered the shock arm more readily and more often, and spent more time in it. In contrast, oxotremorine-treated rats had a stronger memory for the context-footshock association as assessed by all measures of memory. Thus, post-training treatments affecting BLC function modulate memory for Pavlovian contextual fear conditioning in a manner similar to that found with other types of training.
Figures
Fig. 1.
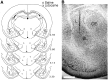
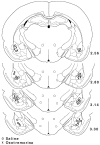
A, A schematic drawing illustrating the injector tip placements of saline-injected rats (circles) and lidocaine-injected rats (crosses). Numbers indicate relative position of the coronal sections in millimeters posterior to bregma (Paxinos and Watson, 1997). B, A microphotograph of a representative injector tip location. L, Lateral nucleus; CE, central nucleus; BL, basolateral/basal nucleus; Pir. Cx, piriform cortex. Scale bar, 0.5 mm.
Fig. 2.
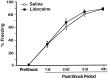
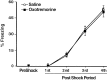
Mean percentage of time spent freezing (±SEM) on day 2 before (PreShock) and during (1st–4th PostShock periods) training in rats that received infusions of saline (open circles) or lidocaine (filled squares) into the BLC after the training.
Fig. 3.
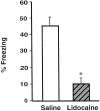
Impaired freezing of rats that had received post-training BLC inactivation. Shown is the mean time spent freezing (±SEM) during the retention test on day 3 in saline-treated rats (open bar) and lidocaine-treated rats (shaded bar). *p < 0.0001 compared with the saline group.
Fig. 4.
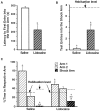
Impaired avoidance of rats that had received post-training BLC inactivation. A, Mean latencies, in seconds, to the first entry into the shock arm (±SEM) during the retention test on day 3 for the saline group (open bar) and the lidocaine group (shaded bar).B, Mean number of entries in the shock arm (±SEM) during the habituation period (dashed lines) and the retention test (bars). C, Mean percentage of time spent per arm during the habituation period on day 1 (dashed line) and the retention test on day 3 (bars; ±SEM). *p < 0.005 compared with the saline group; **p < 0.05 compared with the respective group’s percentage of time in either of the “safe” arms; +, p < 0.005 compared with the habituation period.
Fig. 5.
Injector tip placements for the subjects in the saline group (circles) and the subjects in the oxotremorine group (crosses). _Numbers_indicate relative position of the coronal sections in millimeters posterior to bregma (Paxinos and Watson, 1997).
Fig. 6.
Mean percentage of time spent freezing (±SEM) on day 2 before (PreShock) and during (1st–4th PostShock periods) training in rats that received infusions of saline (open circles) or oxotremorine (filled squares) into the BLC _after_the training.
Fig. 7.
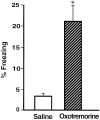
Enhanced freezing of rats that had received muscarinic cholinergic receptor activation of the BLC after the training. Shown is the mean time spent freezing (±SEM) during the retention test on day 3 in rats that received post-training intra-BLC infusions of saline (open bar) or oxotremorine (shaded bar). *p < 0.005 compared with the saline group.
Fig. 8.
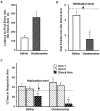
Enhanced avoidance of rats that had received muscarinic cholinergic receptor activation of the BLC after the training. A, Mean latencies, in seconds, to the first entry into the shock arm (±SEM) during the retention test on day 3 for rats in the saline group (open bar) and the oxotremorine group (shaded bar). B, Mean number of entries in the shock arm (±SEM) during the habituation period (dashed line) and the retention test (bars). C, Mean percentage of the time spent per arm during the habituation period on day 1 (dashed line) and the retention test on day 3 (bars; ±SEM). *p < 0.005 compared with the saline group; **p < 0.01 compared with the respective group’s percentage of time in either of the “safe” arms; +,p < 0.0001 compared with the habituation period.
Similar articles
- Post-training intra-basolateral amygdala infusions of norepinephrine enhance consolidation of memory for contextual fear conditioning.
LaLumiere RT, Buen TV, McGaugh JL. LaLumiere RT, et al. J Neurosci. 2003 Jul 30;23(17):6754-8. doi: 10.1523/JNEUROSCI.23-17-06754.2003. J Neurosci. 2003. PMID: 12890768 Free PMC article. - Basolateral amygdala is not critical for cognitive memory of contextual fear conditioning.
Vazdarjanova A, McGaugh JL. Vazdarjanova A, et al. Proc Natl Acad Sci U S A. 1998 Dec 8;95(25):15003-7. doi: 10.1073/pnas.95.25.15003. Proc Natl Acad Sci U S A. 1998. PMID: 9844005 Free PMC article. - Basolateral amygdala inactivation impairs learned (but not innate) fear response in rats.
Ribeiro AM, Barbosa FF, Munguba H, Costa MS, Cavalcante JS, Silva RH. Ribeiro AM, et al. Neurobiol Learn Mem. 2011 May;95(4):433-40. doi: 10.1016/j.nlm.2011.02.004. Epub 2011 Feb 17. Neurobiol Learn Mem. 2011. PMID: 21315824 - Involvement of the basolateral amygdala in muscarinic cholinergic modulation of extinction memory consolidation.
Boccia MM, Blake MG, Baratti CM, McGaugh JL. Boccia MM, et al. Neurobiol Learn Mem. 2009 Jan;91(1):93-7. doi: 10.1016/j.nlm.2008.07.012. Epub 2008 Sep 3. Neurobiol Learn Mem. 2009. PMID: 18706510 Free PMC article. - The basolateral amygdala complex is involved with, but is not necessary for, rapid acquisition of Pavlovian 'fear conditioning'.
Cahill L, Vazdarjanova A, Setlow B. Cahill L, et al. Eur J Neurosci. 2000 Aug;12(8):3044-50. doi: 10.1046/j.1460-9568.2000.00187.x. Eur J Neurosci. 2000. PMID: 10971645
Cited by
- Encoding of emotion-paired spatial stimuli in the rodent hippocampus.
Nalloor R, Bunting KM, Vazdarjanova A. Nalloor R, et al. Front Behav Neurosci. 2012 Jun 14;6:27. doi: 10.3389/fnbeh.2012.00027. eCollection 2012. Front Behav Neurosci. 2012. PMID: 22712009 Free PMC article. - Muscarinic receptors modulate the intrinsic excitability of infralimbic neurons and consolidation of fear extinction.
Santini E, Sepulveda-Orengo M, Porter JT. Santini E, et al. Neuropsychopharmacology. 2012 Aug;37(9):2047-56. doi: 10.1038/npp.2012.52. Epub 2012 Apr 18. Neuropsychopharmacology. 2012. PMID: 22510723 Free PMC article. - Lesions of the nucleus basalis magnocellularis induced by 192 IgG-saporin block memory enhancement with posttraining norepinephrine in the basolateral amygdala.
Power AE, Thal LJ, McGaugh JL. Power AE, et al. Proc Natl Acad Sci U S A. 2002 Feb 19;99(4):2315-9. doi: 10.1073/pnas.022627799. Epub 2002 Feb 5. Proc Natl Acad Sci U S A. 2002. PMID: 11830635 Free PMC article. - Post-training intra-basolateral amygdala infusions of norepinephrine enhance consolidation of memory for contextual fear conditioning.
LaLumiere RT, Buen TV, McGaugh JL. LaLumiere RT, et al. J Neurosci. 2003 Jul 30;23(17):6754-8. doi: 10.1523/JNEUROSCI.23-17-06754.2003. J Neurosci. 2003. PMID: 12890768 Free PMC article. - Intra-amygdala injections of CREB antisense impair inhibitory avoidance memory: role of norepinephrine and acetylcholine.
Canal CE, Chang Q, Gold PE. Canal CE, et al. Learn Mem. 2008 Aug 26;15(9):677-86. doi: 10.1101/lm.904308. Print 2008 Sep. Learn Mem. 2008. PMID: 18772255 Free PMC article.
References
- Albert DJ, Madryga FJ. An examination of the functionally effective spread of four microliters of slowly infused lidocaine. Behav Neural Biol. 1980;29:378–384. - PubMed
- Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. Wiley-Liss; New York: 1992. pp. 1–66.
- Boeijinga PH, Mulder AB, Pennartz CM, Manshanden I, Lopes da Silva FH. Responses of the nucleus accumbens following fornix/fimbria stimulation in the rat. Identification and long-term potentiation of mono- and polysynaptic pathways. Neuroscience. 1993;53:1049–1058. - PubMed
- Brioni JD, McGaugh JL. Post-training administration of GABAergic antagonists enhances retention of aversively motivated tasks. Psychopharmacology (Berl) 1988;96:505–510. - PubMed
- Brioni JD, Nagahara AH, McGaugh JL. Involvement of the amygdala GABAergic system in the modulation of memory storage. Brain Res. 1989;487:105–112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical