CTLA-4 blockade enhances the immune response induced by mycobacterial infection but does not lead to increased protection - PubMed (original) (raw)
CTLA-4 blockade enhances the immune response induced by mycobacterial infection but does not lead to increased protection
J Kirman et al. Infect Immun. 1999 Aug.
Abstract
The murine immune response to a pulmonary mycobacterial infection is slow to develop, allowing bacterial numbers to increase in the lung for several weeks after infection. We sought to enhance the protective immune response induced during Mycobacterium bovis BCG infection by administering an antibody that blocks the interaction of CTLA-4 with its ligands, CD80 and CD86. We found that injection of anti-CTLA-4 monoclonal antibody (MAb) greatly enhanced and accelerated the immune response, as measured by increased cellularity of the draining mediastinal lymph nodes, and enhanced antigen-inducible proliferation and gamma interferon production by mediastinal lymphocytes in vitro. However, despite the apparently enhanced immune response in the mediastinal lymph node following treatment with anti-CTLA-4 MAb, there was no improvement in clearance of mycobacteria in the lungs, liver, or spleen. Examination of the primary site of infection, the lung, revealed that CTLA-4 blockade had no effect on the number or function of lymphocytes infiltrating the infected lung tissue. Taken together, these data suggest that in vivo CTLA-4 blockade enhances mycobacterial-infection-induced lymphocyte expansion and effector cell cytokine production in the draining lymph node but does not alter the number or function of lymphocytes at the primary site of infection and therefore does not lead to enhanced clearance of the infection.
Figures
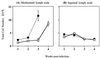
FIG. 1
Treatment with anti-CTLA-4 MAb increases total lymphocyte numbers in the draining mediastinal lymph nodes of BCG-infected mice. C57BL/6 mice were intranasally infected with 5 × 105 BCG and either treated with anti-CTLA-4 MAb (solid squares) or left untreated (open squares). The draining mediastinal lymph nodes (A) or nondraining inguinal lymph nodes (B) were collected at the times indicated. Single-cell suspensions were prepared from individual lymph nodes, and total lymphocytes were counted. The values represent mean cell numbers from four mice ± standard error, and the results are representative of three separate experiments.
FIG. 2
Treatment with anti-CTLA-4 MAb does not alter BCG-induced changes in mediastinal lymph node cell populations. C57BL/6 mice were left uninfected (open circles) or intranasally infected with BCG and either treated with anti-CTLA-4 MAb (solid squares) or left untreated (open squares). Mediastinal lymphocytes were stained for fluorescence-activated cell sorter analysis with anti-CD4, -CD8, or -B220 MAbs. The values represent the percentage of positive cells from lymphocytes pooled from four mice. The results shown are representative of two separate experiments.
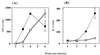
FIG. 3
Treatment with anti-CTLA-4 MAb in vivo enhances in vitro antigen-specific proliferation of mediastinal lymphocytes. C57BL/6 mice were intranasally infected with 5 × 104 (A and B) or 5 × 105 (C and D) BCG and either treated with anti-CTLA-4 MAb or left untreated (controls). At the indicated times postinfection, total lymphocytes from the mediastinal lymph node (A and C) or inguinal lymph nodes (B and D) were stimulated in vitro with either BCG-pulsed or unpulsed peritoneal macrophages. Cultures were incubated for 72 h, and proliferation was measured by [3H]thymidine incorporation in the last 10 h of culture. The values at 2 weeks postinfection represent the mean counts per minute (CPM) of lymphocytes pooled from four mice ± standard error (SE). The values at other time points represent the mean CPM of separate samples from four mice ± SE. APC, antigen-presenting cells.
FIG. 4
In vivo treatment with anti-CTLA-4 MAb enhances in vitro cytokine production. C57BL/6 mice were intranasally infected with 5 × 104 BCG and treated with either anti-CTLA-4 MAb (solid squares) or left untreated (open squares). (A) At the indicated times postinfection, total lymph node cells from the draining mediastinal lymph nodes were stimulated in vitro with C57BL/6 peritoneal-derived macrophages pulsed with BCG. Supernatants were harvested after 72 h, and their IFN-γ content was measured by ELISA. The values at 2 weeks postinfection represent the mean IFN-γ production of lymphocytes pooled from four mice ± standard error (SE). The values at other time points represent the mean IFN-γ production of separate samples from four mice ± SE. (B) At the indicated times postinfection, total lymph node cells from the draining mediastinal lymph nodes were stimulated in vitro with immobilized anti-CD3 MAb. IL-5 levels in the supernatant after 48 h were measured by ELISA. The values at 1 and 2 weeks postinfection represent the mean IL-5 production of lymphocytes pooled from four mice ± SE. The values at other time points represent the mean IL-5 production of separate samples from four mice ± SE.
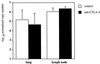
FIG. 5
Treatment with anti-CTLA-4 MAb has no detectable effect on IFN-γ gene expression in the mediastinal lymph nodes and lungs of BCG-infected mice. C57BL/6 mice were intranasally infected with 5 × 105 BCG and either treated with anti-CTLA-4 MAb (solid bars) or left untreated (open bars). The mice were sacrificed at 3 weeks after infection, and the mediastinal lymph nodes and lungs were collected and analyzed for IFN-γ mRNA levels by a real-time quantitative PCR method. The values were normalized to IFN-β2m, and represent the geometric mean of CFU from four mice ± standard error.
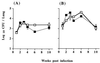
FIG. 6
Treatment with anti-CTLA-4 MAb has no detectable effect on the growth of BCG in the lung. C57BL/6 mice were intranasally infected with 5 × 104 (A) or 5 × 105 (B) BCG and either treated with anti-CTLA-4 MAb (solid squares) or left untreated (open squares). The mice were sacrificed at the indicated time points after infection, and the lungs were removed, homogenized, and plated on Middlebrook 7H11 agar for viable bacterial counts. The values represent the geometric mean of CFU from four mice ± standard error.
FIG. 7
Treatment with anti-CTLA-4 MAb does not affect granuloma formation in tissues. C57BL/6 mice were intranasally infected with 5 × 105 BCG and either treated with anti-CTLA-4 MAb (A) or left untreated (B). After 3 weeks of infection, the lungs were removed for histological analysis, fixed in formalin, and embedded in paraffin wax. Sections (3 μm) were stained with hematoxylin and eosin, and sections were analyzed by light microscopy (magnification, ×190). The results shown are representative of three separate experiments.
Similar articles
- Protective immunity to nematode infection is induced by CTLA-4 blockade.
McCoy K, Camberis M, Gros GL. McCoy K, et al. J Exp Med. 1997 Jul 21;186(2):183-7. doi: 10.1084/jem.186.2.183. J Exp Med. 1997. PMID: 9221747 Free PMC article. - Enhanced induction of antitumor T-cell responses by cytotoxic T lymphocyte-associated molecule-4 blockade: the effect is manifested only at the restricted tumor-bearing stages.
Yang YF, Zou JP, Mu J, Wijesuriya R, Ono S, Walunas T, Bluestone J, Fujiwara H, Hamaoka T. Yang YF, et al. Cancer Res. 1997 Sep 15;57(18):4036-41. Cancer Res. 1997. PMID: 9307290 - Pulmonary immune responses during primary mycobacterium bovis- Calmette-Guerin bacillus infection in C57Bl/6 mice.
Fulton SA, Martin TD, Redline RW, Henry Boom W. Fulton SA, et al. Am J Respir Cell Mol Biol. 2000 Mar;22(3):333-43. doi: 10.1165/ajrcmb.22.3.3776. Am J Respir Cell Mol Biol. 2000. PMID: 10696070 - CTLA-4 blockade enhances clinical disease and cytokine production during experimental allergic encephalomyelitis.
Perrin PJ, Maldonado JH, Davis TA, June CH, Racke MK. Perrin PJ, et al. J Immunol. 1996 Aug 15;157(4):1333-6. J Immunol. 1996. PMID: 8759711 - [Regulation of T-cell activation by CD28 and CTLA-4].
Nagel T, Kalden JR, Manger B. Nagel T, et al. Med Klin (Munich). 1998 Oct 15;93(10):592-7. doi: 10.1007/BF03042674. Med Klin (Munich). 1998. PMID: 9849050 Review. German.
Cited by
- A narrative review of the controversy on the risk of mycobacterial infections with immune checkpoint inhibitor use: does Goldilocks have the answer?
Vaddi A, Hulsebus HJ, O'Neill EL, Knight V, Chan ED. Vaddi A, et al. J Thorac Dis. 2024 Feb 29;16(2):1601-1624. doi: 10.21037/jtd-23-1395. Epub 2024 Feb 27. J Thorac Dis. 2024. PMID: 38505086 Free PMC article. Review. - The paradox of immune checkpoint inhibition re-activating tuberculosis.
Ahmed M, Tezera LB, Elkington PT, Leslie AJ. Ahmed M, et al. Eur Respir J. 2022 Nov 10;60(5):2102512. doi: 10.1183/13993003.02512-2021. Print 2022 Nov. Eur Respir J. 2022. PMID: 35595321 Free PMC article. Review. - CD84 is a Suppressor of T and B Cell Activation during Mycobacterium tuberculosis Pathogenesis.
Zheng N, Fleming J, Hu P, Jiao J, Zhang G, Yang R, Li C, Liu Y, Bi L, Zhang H. Zheng N, et al. Microbiol Spectr. 2022 Feb 23;10(1):e0155721. doi: 10.1128/spectrum.01557-21. Epub 2022 Feb 23. Microbiol Spectr. 2022. PMID: 35196822 Free PMC article. - Decreased Expression of CD69 on T Cells in Tuberculosis Infection Resisters.
Chen ZY, Wang L, Gu L, Qu R, Lowrie DB, Hu Z, Sha W, Fan XY. Chen ZY, et al. Front Microbiol. 2020 Aug 7;11:1901. doi: 10.3389/fmicb.2020.01901. eCollection 2020. Front Microbiol. 2020. PMID: 32849474 Free PMC article. - T cell costimulation, checkpoint inhibitors and anti-tumor therapy.
Nandi D, Pathak S, Verma T, Singh M, Chattopadhyay A, Thakur S, Raghavan A, Gokhroo A, Vijayamahantesh. Nandi D, et al. J Biosci. 2020;45:50. J Biosci. 2020. PMID: 32345776 Review.
References
- Brown I N. Animal models and immune mechanisms in mycobacterial infection. In: Ratledge C, Stanford J, editors. Biology of mycobacteria. Vol. 2. London, England: Academic Press; 1983. pp. 173–233.
- Brunet J F, Denizot F, Luciani M F, Roux-Dosseto M, Suzan M, Mattei M G, Golstein P. A new member of the immunoglobulin superfamily—CTLA-4. Nature. 1987;328:267–270. - PubMed
- Denis M. Involvement of cytokines in determining resistance and acquired immunity in murine tuberculosis. J Leukoc Biol. 1991;50:495–501. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical