Wnt-induced dephosphorylation of axin releases beta-catenin from the axin complex - PubMed (original) (raw)
Wnt-induced dephosphorylation of axin releases beta-catenin from the axin complex
K Willert et al. Genes Dev. 1999.
Abstract
The stabilization of beta-catenin is a key regulatory step during cell fate changes and transformations to tumor cells. Several interacting proteins, including Axin, APC, and the protein kinase GSK-3beta are implicated in regulating beta-catenin phosphorylation and its subsequent degradation. Wnt signaling stabilizes beta-catenin, but it was not clear whether and how Wnt signaling regulates the beta-catenin complex. Here we show that Axin is dephosphorylated in response to Wnt signaling. The dephosphorylated Axin binds beta-catenin less efficiently than the phosphorylated form. Thus, Wnt signaling lowers Axin's affinity for beta-catenin, thereby disengaging beta-catenin from the degradation machinery.
Figures
Figure 1
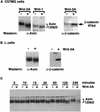
Wnt signaling modifies Axin. (A) Western analysis of Axin and β-catenin protein from C57MG cells treated with either Wnt-1 or Wnt-3A. (B) Axin and β-catenin protein from L cells treated with Wnt-3A. C57MG and L cells were stimulated with control (−) or Wnt-3A (+)-conditioned medium for 2 hr prior to lysis. C57MG cells containing a tet-repressible Wnt-1 transgene were incubated in tet-containing medium to repress Wnt-1 expression (−) or in tet-free medium for 12 hr to induce Wnt-1 expression (+). (C) Time course of Axin protein modification in response to Wnt-3A. C57MG cells were stimulated with control (−) or Wnt-3A (+)-conditioned medium for the indicated times and immunoblotted with anti-Axin antibody.
Figure 2
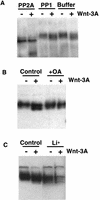
The Axin protein is phosphorylated. (A) PP2A, but not PP1, dephosphorylates the Axin protein in vitro. Axin protein was immunoprecipitated from cell extracts of C57MG cells treated with control (−) or Wnt-3A (+)-conditioned medium. The immune complexes were treated with phosphatase buffer only, PP2A, or PP1 and immunoblotted with anti-Axin antibody. (B) The phosphatase inhibitor okadaic acid inhibits the dephosphorylation of Axin. Cell lysates were prepared from C57MG cells treated with control (−) or Wnt-3A (+)-conditioned medium and with or without 400 n
m
okadaic acid and immunoblotted with anti-Axin antibody. (C) Lithium, an inhibitor of GSK-3β, promotes the dephosphorylation of Axin. Cell lysates were prepared from C57MG cells first treated with or without 25 m
m
LiCl for 4 hr and treated with control (−) or Wnt3A (+)-conditioned medium for an additional 2 hr.
Figure 3
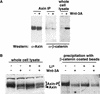
In the absence of a Wnt signal, Axin binds β-catenin with higher affinity. (A) β-Catenin coimmunoprecipitates with Axin. Axin protein was immunoprecipitated from L cells treated with control (−) or Wnt-3A (+)-conditioned medium and immunoblotted with either anti-Axin or anti-β-catenin antibodies. (B) Axin from Wnt-3A-stimulated cells binds β-catenin less efficiently. β-Catenin-coated beads were used to retrieve the Axin protein from cell extracts prepared from C57MG cells treated with control (−) or Wnt-3A (+)-conditioned medium in the absence (−) or presence (+) of 25 m
m
LiCl. The precipitating Axin protein was then visualized by immunoblotting with anti-Axin antibodies. The bracket indicates the position of phosphorylated Axin (Axin-P); the arrowhead indicates the position of un- or under-phosphorylated Axin (Axin).
Figure 4
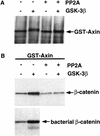
Phosphorylation of Axin with GSK-3β increases Axin’s affinity for β-catenin. (A) Phosphorylation of GST–Axin with GSK-3β and subsequent dephosphorylation with PP2A. GST–Axin immobilized on glutathione–Sepharose beads was treated with (+) or without (−) purified GSK-3β. Phosphorylated or unphosphorylated GST–Axin subsequently was washed to remove the ATP and incubated in the absence (−) or presence (+) of purified PP2A. The GST–Axin protein was visualized by immunoblotting with anti-Axin antibody. (B) GSK-3β phosphorylated Axin binds β-catenin significantly better than unphosphorylated or dephosphorylated GST–Axin. The GSK-3β and PP2A-modified GST–Axin proteins were used to precipitate β-catenin from lysates of L cells treated with Wnt-3A. Phosphorylated and unphosphorylated GST–Axin was also used to precipitate bacterially produced β-catenin protein. Axin-binding proteins were immunoblotted with anti-β-catenin antibody.
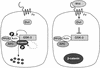
Figure 5
A model for the role of Axin in Wnt signal transduction. In an unstimulated cell, GSK-3β is active and phosphorylates Axin, which in turn, recruits β-catenin into the Axin/GSK-3β complex. By virtue of its proximity to GSK-3β, β-catenin is then phosphorylated. Phosphorylated β-catenin is then targeted for degradation. Upon transduction of the Wnt signal through the Frizzled (Fz) receptors to Dishevelled (Dvl), GSK-3β kinase activity is inhibited so that PP2A dephosphorylates Axin. Unphosphorylated Axin, in turn, no longer recruits β-catenin to the complex. Failure of β-catenin to associate with the Axin/GSK-3β complex prevents its phosphorylation by GSK-3β so that it can accumulate to high levels in the cytoplasm and nucleus and activate transcription in concert with the Tcf/Lef-1 family of transcription factors. GSK-3β also phosphorylates APC, which may facilitate β-catenin recruitment into the complex; however, this event has not been shown to be regulated by Wnt signaling.
Similar articles
- Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin.
Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Ikeda S, et al. EMBO J. 1998 Mar 2;17(5):1371-84. doi: 10.1093/emboj/17.5.1371. EMBO J. 1998. PMID: 9482734 Free PMC article. - Axin, an inhibitor of the Wnt signalling pathway, interacts with beta-catenin, GSK-3beta and APC and reduces the beta-catenin level.
Nakamura T, Hamada F, Ishidate T, Anai K, Kawahara K, Toyoshima K, Akiyama T. Nakamura T, et al. Genes Cells. 1998 Jun;3(6):395-403. doi: 10.1046/j.1365-2443.1998.00198.x. Genes Cells. 1998. PMID: 9734785 - Bridging of beta-catenin and glycogen synthase kinase-3beta by axin and inhibition of beta-catenin-mediated transcription.
Sakanaka C, Weiss JB, Williams LT. Sakanaka C, et al. Proc Natl Acad Sci U S A. 1998 Mar 17;95(6):3020-3. doi: 10.1073/pnas.95.6.3020. Proc Natl Acad Sci U S A. 1998. PMID: 9501208 Free PMC article. - Modulation of Wnt signaling by Axin and Axil.
Kikuchi A. Kikuchi A. Cytokine Growth Factor Rev. 1999 Sep-Dec;10(3-4):255-65. doi: 10.1016/s1359-6101(99)00017-9. Cytokine Growth Factor Rev. 1999. PMID: 10647780 Review. - New steps in the Wnt/beta-catenin signal transduction pathway.
Sakanaka C, Sun TQ, Williams LT. Sakanaka C, et al. Recent Prog Horm Res. 2000;55:225-36. Recent Prog Horm Res. 2000. PMID: 11036939 Review.
Cited by
- Biochemical characterization of the Drosophila wingless signaling pathway based on RNA interference.
Matsubayashi H, Sese S, Lee JS, Shirakawa T, Iwatsubo T, Tomita T, Yanagawa S. Matsubayashi H, et al. Mol Cell Biol. 2004 Mar;24(5):2012-24. doi: 10.1128/MCB.24.5.2012-2024.2004. Mol Cell Biol. 2004. PMID: 14966281 Free PMC article. - The various roles of ubiquitin in Wnt pathway regulation.
Tauriello DV, Maurice MM. Tauriello DV, et al. Cell Cycle. 2010 Sep 15;9(18):3700-9. doi: 10.4161/cc.9.18.13204. Epub 2010 Sep 25. Cell Cycle. 2010. PMID: 20930545 Free PMC article. Review. - Colocalization and redistribution of dishevelled and actin during Wnt-induced mesenchymal morphogenesis.
Torres MA, Nelson WJ. Torres MA, et al. J Cell Biol. 2000 Jun 26;149(7):1433-42. doi: 10.1083/jcb.149.7.1433. J Cell Biol. 2000. PMID: 10871283 Free PMC article. - Axin Family of Scaffolding Proteins in Development: Lessons from C. elegans.
Mallick A, Taylor SKB, Ranawade A, Gupta BP. Mallick A, et al. J Dev Biol. 2019 Oct 15;7(4):20. doi: 10.3390/jdb7040020. J Dev Biol. 2019. PMID: 31618970 Free PMC article. Review. - Toggling a conformational switch in Wnt/β-catenin signaling: regulation of Axin phosphorylation. The phosphorylation state of Axin controls its scaffold function in two Wnt pathway protein complexes.
Tacchelly-Benites O, Wang Z, Yang E, Lee E, Ahmed Y. Tacchelly-Benites O, et al. Bioessays. 2013 Dec;35(12):1063-70. doi: 10.1002/bies.201300101. Epub 2013 Sep 19. Bioessays. 2013. PMID: 24105937 Free PMC article. Review.
References
- Behrens J, Jerchow BA, Wurtele M, Grimm J, Asbrand C, Wirtz R, Kuhl M, Wedlich D, Birchmeier W. Functional interaction of an axin homolog, conductin, with β-catenin, APC, and GSK3β. Science. 1998;280:596–599. - PubMed
- Brown JD, Moon RT. Wnt signaling: Why is everything so negative? Curr Opin Cell Biol. 1998;10:182–187. - PubMed
- Cadigan KM, Nusse R. Wnt signaling: A common theme in animal development. Genes & Dev. 1997;11:3286–3305. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases