Depth penetration and detection of pH gradients in biofilms by two-photon excitation microscopy - PubMed (original) (raw)
Comparative Study
Depth penetration and detection of pH gradients in biofilms by two-photon excitation microscopy
J M Vroom et al. Appl Environ Microbiol. 1999 Aug.
Abstract
Deep microbial biofilms are a major problem in many industrial, environmental, and medical settings. Novel approaches are needed to understand the structure and metabolism of these biofilms. Two-photon excitation microscopy (TPE) and conventional confocal laser scanning microscopy (CLSM) were compared quantitatively for the ability to visualize bacteria within deep in vitro biofilms. pH gradients within these biofilms were determined by fluorescence lifetime imaging, together with TPE. A constant-depth film fermentor (CDFF) was inoculated for 8 h at 50 ml. h(-1) with a defined mixed culture of 10 species of bacteria grown in continuous culture. Biofilms of fixed depths were developed in the CDFF for 10 or 11 days. The microbial compositions of the biofilms were determined by using viable counts on selective and nonselective agar media; diverse mixed-culture biofilms developed, including aerobic, facultative, and anaerobic species. TPE was able to record images four times deeper than CLSM. Importantly, in contrast to CLSM images, TPE images recorded deep within the biofilm showed no loss of contrast. The pH within the biofilms was measured directly by means of fluorescence lifetime imaging; the fluorescence decay of carboxyfluorescein was correlated with biofilm pH and was used to construct a calibration curve. pH gradients were detectable, in both the lateral and axial directions, in steady-state biofilms. When biofilms were overlaid with 14 mM sucrose for 1 h, distinct pH gradients developed. Microcolonies with pH values of below pH 3.0 were visible, in some cases adjacent to areas with a much higher pH (>5.0). TPE allowed resolution of images at significantly greater depths (as deep as 140 microm) than were possible with CLSM. Fluorescence lifetime imaging allowed the in situ, real-time imaging of pH and the detection of sharp gradients of pH within microbial biofilms.
Figures
FIG. 1
CDFF apparatus, modified glass coverslip assembly, and imaging.
FIG. 2
Time-gated fluorescence lifetime imaging, in which the sample is excited with a brief excitation pulse, after which the fluorescence intensity is recorded in two (or more) time gates.
FIG. 3
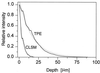
Fluorescence penetration depth in xz sections of rhodamine B-stained biofilm by confocal (single-photon) imaging (a) and TPE (b). Depths are measured from the biofilm-coverslip interface.
FIG. 4
Axial (xz) intensity profiles derived from Fig. 3.
FIG. 5
Fluorescence xy images of 30 by 30 μm recorded at distances of 10, 40, and 60 μm from the biofilm-coverslip interface (see Fig. 1) by using CLSM (right) and TPE (left).
FIG. 6
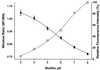
Solid line, relation between biofilm pH and window ratio (_W_1/_W_4), with data points fitted to a Boltzmann sigmoidal curve: y = [(_A_1 _A_2)/(1 + e(x − _x_50)/dx)] + _A_2, where _A_1 and _A_2 are the asymptotes, _x_50 is the center, and dx is the width (χ2 = 3 × 10−5). The error bars reflect the variability (standard deviation) of three independent calibration experiments. Dashed line, relative fluorescence intensity of carboxyfluorescein as a function of pH.
FIG. 7
TPE xy images of biofilms at 5 μm (A) and 70 μm (C) from the biofilm-coverslip interface. (B) pH image of panel A. Bar, 5 μm.
FIG. 8
Window ratio (pH) image of an xz section through biofilm. A higher intensity (lighter image) in the window ratio images indicates a lower pH value. The top of the image is the biofilm-buffer interface; the bottom represents the biofilm-coverslip interface.
FIG. 9
Intensity (A and B) and window ratio (pH) (C and D) images in biofilm, before (A and C) and 60 min after (B and D) addition of a 14 mM sucrose solution. Images were recorded at 65 μm from the surface. The average pH values are 6.2 for panel C and 5.5 for panel D. Bar, 10 μm.
FIG. 10
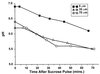
Decrease in mean biofilm pH in a 100-μm biofilm following a sucrose pulse. The average pH over 30-by 30-μm xy images (100 by 100 pixels) at 70, 35, and 5 μm from the biofilm-coverslip assembly is shown.
Similar articles
- Imaging of optically thick specimen using two-photon excitation microscopy.
Gerritsen HC, De Grauw CJ. Gerritsen HC, et al. Microsc Res Tech. 1999 Nov 1;47(3):206-9. doi: 10.1002/(SICI)1097-0029(19991101)47:3<206::AID-JEMT6>3.0.CO;2-H. Microsc Res Tech. 1999. PMID: 10544335 - Combined pH ratiometry and fluorescence lectin-binding analysis (pH-FLBA) for microscopy-based analyses of biofilm pH and matrix carbohydrates.
Del Rey YC, Schramm A, L Meyer R, Lund MB, Schlafer S. Del Rey YC, et al. Appl Environ Microbiol. 2024 Feb 21;90(2):e0200723. doi: 10.1128/aem.02007-23. Epub 2024 Jan 24. Appl Environ Microbiol. 2024. PMID: 38265212 Free PMC article. - Demineralization of dentin by Streptococcus mutans biofilms grown in the constant depth film fermentor.
Deng DM, ten Cate JM. Deng DM, et al. Caries Res. 2004 Jan-Feb;38(1):54-61. doi: 10.1159/000073921. Caries Res. 2004. PMID: 14684978 - Two-photon excitation of fluorescence for three-dimensional optical imaging of biological structures.
Diaspro A, Robello M. Diaspro A, et al. J Photochem Photobiol B. 2000 Mar;55(1):1-8. doi: 10.1016/s1011-1344(00)00028-2. J Photochem Photobiol B. 2000. PMID: 10877060 Review. - Two-photon excitation fluorescence microscopy.
So PT, Dong CY, Masters BR, Berland KM. So PT, et al. Annu Rev Biomed Eng. 2000;2:399-429. doi: 10.1146/annurev.bioeng.2.1.399. Annu Rev Biomed Eng. 2000. PMID: 11701518 Review.
Cited by
- Viscoelasticity of biofilms and their recalcitrance to mechanical and chemical challenges.
Peterson BW, He Y, Ren Y, Zerdoum A, Libera MR, Sharma PK, van Winkelhoff AJ, Neut D, Stoodley P, van der Mei HC, Busscher HJ. Peterson BW, et al. FEMS Microbiol Rev. 2015 Mar;39(2):234-45. doi: 10.1093/femsre/fuu008. Epub 2015 Feb 2. FEMS Microbiol Rev. 2015. PMID: 25725015 Free PMC article. Review. - pH variation in medical implant biofilms: Causes, measurements, and its implications for antibiotic resistance.
Behbahani SB, Kiridena SD, Wijayaratna UN, Taylor C, Anker JN, Tzeng TJ. Behbahani SB, et al. Front Microbiol. 2022 Oct 31;13:1028560. doi: 10.3389/fmicb.2022.1028560. eCollection 2022. Front Microbiol. 2022. PMID: 36386694 Free PMC article. Review. - Simultaneous spatiotemporal mapping of in situ pH and bacterial activity within an intact 3D microcolony structure.
Hwang G, Liu Y, Kim D, Sun V, Aviles-Reyes A, Kajfasz JK, Lemos JA, Koo H. Hwang G, et al. Sci Rep. 2016 Sep 8;6:32841. doi: 10.1038/srep32841. Sci Rep. 2016. PMID: 27604325 Free PMC article. - Antimicrobial action of carvacrol at different stages of dual-species biofilm development by Staphylococcus aureus and Salmonella enterica serovar Typhimurium.
Knowles JR, Roller S, Murray DB, Naidu AS. Knowles JR, et al. Appl Environ Microbiol. 2005 Feb;71(2):797-803. doi: 10.1128/AEM.71.2.797-803.2005. Appl Environ Microbiol. 2005. PMID: 15691933 Free PMC article. - The exopolysaccharide matrix: a virulence determinant of cariogenic biofilm.
Koo H, Falsetta ML, Klein MI. Koo H, et al. J Dent Res. 2013 Dec;92(12):1065-73. doi: 10.1177/0022034513504218. Epub 2013 Sep 17. J Dent Res. 2013. PMID: 24045647 Free PMC article. Review.
References
- Baelum V, Fejerskov O, Küseler A. Approximal plaque pH following topical applications of standard buffers in vivo. Caries Res. 1994;28:116–122. - PubMed
- Barber P, Boyd A, Newman H N, Challacombe S J, Vrahopoulos T P, Gill S. Immunogold labelling of Porphyromonas gingivalis in pure culture and in apical border plaque. Microb Ecol Health Dis. 1990;3:217–222.
- Brading M, Jass J, Lappin-Scott H M. Dynamics of bacterial biofilm formation. In: Lappin-Scott H M, Costerton J W, editors. Plant and microbial biotechnology research series, no. 5. Microbial biofilms. Cambridge, United Kingdom: Cambridge University Press; 1995. pp. 46–63.
- Bradshaw D J, McKee A S, Marsh P D. Effects of carbohydrate pulses and pH on population shifts within oral microbial communities in vitro. J Dent Res. 1989;68:1298–1302. - PubMed
- Buurman E P, Sanders R, Draaijer A, Gerritsen H C, van Veen J J F, Houpt P M, Levine Y K. Fluorescence lifetime imaging using a confocal laser scanning microscope. Scanning. 1992;14:152–159.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources