The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis - PubMed (original) (raw)
The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis
M D Sternlicht et al. Cell. 1999.
Abstract
Matrix metalloproteinases (MMPs) are invariably upregulated in the stromal compartment of epithelial cancers and appear to promote invasion and metastasis. Here we report that phenotypically normal mammary epithelial cells with tetracycline-regulated expression of MMP3/stromelysin-1 (Str1) form epithelial glandular structures in vivo without Str1 but form invasive mesenchymal-like tumors with Str1. Once initiated, the tumors become independent of continued Str1 expression. Str1 also promotes spontaneous premalignant changes and malignant conversion in mammary glands of transgenic mice. These changes are blocked by coexpression of a TIMP1 transgene. The premalignant and malignant lesions have stereotyped genomic changes unlike those seen in other murine mammary cancer models. These data indicate that Str1 influences tumor initiation and alters neoplastic risk.
Figures
Figure 1. Effect of Str1 on Morphology and Intermediate Filament Expression in Scp2 Cells
Cells were maintained for 6 days in the (a) absence or (b) presence of activated recombinant Str1 (rStr1) and stained by indirect immunofluorescence for cytokeratins (red) and vimentin (green). Nuclei were counterstained with DAPI (blue). Scale bar, 50 µm.
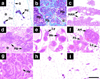
Figure 2. Effect of Str1 on Mammary Epithelial Growth In Vivo
(A) Histologic appearance of p2S10 mammary epithelial cells grown in cleared mammary fat pads. (a–d) Appearance of epithelial ductal and gland-like structures (Ep) that form in the absence of Str1 expression as seen by (a) whole-mount, (b) H&E, (c) anti-cytokeratin-8, and (d) anti-smooth muscle actin staining. The arrowhead in (d) indicates vascular smooth muscle cells. (e–h) Appearance of spindle-cell tumors (Sp) that form when Str1 expression is induced in vivo or prior to injection as seen by (e) H&E, (f) Alcian blue, (g) anti-vimentin, and (h) anti-cytokeratin-8 staining. Areas of cartilage (chondroid metaplasia, Cm) are present in the upper right corner (e–h). Scale bars, (a) 200 µm and (b–h) 100 µm. (B) Tumor incidence (percent of injected sites) and volume (cm3; mean ± SEM) following injection of parental (Scp2), nonexpressing (p2S3), uninduced (S10un), and preinduced (S10pre) cells into cleared mammary fat pads in mice maintained for 6 weeks with (+) or without (−) Tet in their drinking water or without Tet for the first 12 days only (−/+). *, p < 0.0001 versus S10pre cells in mice maintained with or without Tet (two-tailed Fisher’s exact test); †, p < 0.05 versus S10un cells in mice maintained with Tet and p < 0.001 versus S10pre cells in mice maintained with or without Tet (Fisher’s exact test); ‡, p < 0.005 and p < 0.001 versus S10pre cells in mice maintained with and without Tet, respectively (t test).
Figure 3. Incidence of Mammary Gland Pathologies in Str1 Transgenic Mice
The _Str1_-expressing transgenics include 100, 31, 16, 9, and 7 mice from five independent transgenic lines, respectively. *, p < 0.00001 versus nontransgenic or nonexpressing transgenic controls (two-tailed Fisher’s exact test); †, p = 0.002 and p = 0.02 versus non-transgenic and nonexpressing transgenic controls, respectively (Fisher’s exact test).
Figure 4. Histologic Appearance of Normal and WAP-Str1 Mammary Glands
Histologic sections are from (a) nontransgenic, (b–g) WAP-Str1 transgene–expressing, (h) WAP-Str1 transgene–nonexpressing, and (i) Str1/TIMP1 double transgene–positive female mice. (a) Normal mammary gland with resting ducts (Du), abundant adipose tissue (asterisk), and minimal periductal (Pd) and septal (S) collagen (stained blue). (b) Severe hyperplasia (Hp) with considerable intervening fibrosis (Fb; stained blue) and multilocular adipocytes (asterisk). (c) Hyperplastic alveolar nodule (HAN) with lipid droplets characteristic of secretory activity even though this gland comes from a nulliparous mouse. (d) Multifocal alveolar hyperplasia (Hp) with eosinophilic (pink) fibrotic areas and multilocular adipocytes (asterisk). (e) Intraductal papillary hyperplasia with lymphocytic infiltrates (Ly). The small hyperchromatic cells (Me) were cytokeratin-8 negative and smooth muscle actin positive, indicating the abnormal presence of myoepithelial cells within the severely distended ducts. (f) Atypical hyperplasia (AH) with lymphocytic infiltrates (Ly) and mild fibrosis (Fb). (g) Atypical hyperplasia with considerable fibrosis. (h and i) Normal mammary histology seen with the loss of Str1 transgene expression or its inhibition by TIMP1, respectively. (a and b) Masson’s trichrome. (c–i) Hematoxylin/eosin. Scale bar, 200 µm.
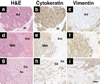
Figure 5. Histologic Appearance of Malignant Mammary Tumors from Str1 Transgenic Mice
(a–c) Moderately differentiated adenocarcinoma (Ad) with adjacent and intervening vimentin-positive stromal cells (St). (d–f) Renal metastasis (Met) from an undifferentiated mammary carcinoma. Normal kidney (Kid) is present on the right. (g–i) Carcinosarcoma with distinct carcinomatous (Ca) and sarcomatous (Sa) areas exhibiting epithelial and mesenchymal features, respectively. H&E, hematoxylin/eosin stains. Scale bar, 200 µm.
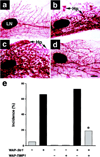
Figure 6. Effect of a TIMP1 Transgene on Str1 Transgene–Induced Mammary Hyperplasias as Seen by Whole-Mount Staining
(a) 16-month-old nontransgenic control. (b) 16-month-old WAP-Str1 transgenic mouse with multifocal alveolar hyperplasia (Hp). (c) 12-month-old WAP-_Str1_-positive/WAP-_TIMP1_-negative mouse with diffuse alveolar hyperplasia. (d) 12-month-old Str1/TIMP1 double transgenic mouse from the same litter as the mouse in (c). The mammary gland in (d) was judged to be within normal limits by whole-mount and hematoxylin/eosin staining. The glands shown are from mice that had undergone a single pregnancy and lactation at least 5 months prior to sacrifice. LN, lymph node. Scale bar, 500 µm. (e) Incidence of mammary hyperplasias in double and single transgenic mice. The single transgenic mice are from the related M2-5 line. Gray and black bars indicate mild and moderate to severe hyperplasias, respectively. *, p < 0.02 versus littermates carrying the Str1 transgene alone and p < 0.0006 versus the large cohort of M2-5 Str1 transgenics, respectively (two-tailed Fisher’s exact test).
Figure 7. CGH Profiles
(A) Genomic changes seen in the mammary glands of 12 individual Str1 transgenic mice. Samples 1, 3, 10, and 11 were from one transgenic founder line; samples 4 and 8 were from another line; and the remaining samples were from a third independent line. Approximate locations of macroscopic DNA gains (green) and losses (red) are indicated along otherwise unaltered (yellow) chromosomes, with black circles representing acrocentric centromeres. Sample 12, a carcinosarcoma, was microdissected and its carcinomatous and sarcomatous regions analyzed separately. All adjacent stromal and nonmammary control tissues had normal CGH profiles. (B) Normalized fluorescence intensity profiles for chromosome 15 obtained with DNA isolated from (a) parental Scp2 cells, (b) a p2S7 cell–derived tumor, (c) preinduced p2S10 cells, (d) microdissected spindle-cell areas from a tumor derived from the same preinduced cells as in (c), (e) chondroid areas from the same tumor as in (d), and (f) normal stroma adjacent to the tumor in (d) and (e). Average green:red fluorescence ratios (heavy lines) ± 1 standard deviation (thin lines) are shown for the number of metaphase chromosomes examined (n). Dashed horizontal lines and upper and lower dotted lines indicate fluorescence ratios of 1, 1.5, and 0.5, respectively.
Similar articles
- Stromelysin-1 regulates adipogenesis during mammary gland involution.
Alexander CM, Selvarajan S, Mudgett J, Werb Z. Alexander CM, et al. J Cell Biol. 2001 Feb 19;152(4):693-703. doi: 10.1083/jcb.152.4.693. J Cell Biol. 2001. PMID: 11266461 Free PMC article. - The matrix metalloproteinase stromelysin-1 acts as a natural mammary tumor promoter.
Sternlicht MD, Bissell MJ, Werb Z. Sternlicht MD, et al. Oncogene. 2000 Feb 21;19(8):1102-13. doi: 10.1038/sj.onc.1203347. Oncogene. 2000. PMID: 10713697 Free PMC article. Review. - Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells.
Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ. Lochter A, et al. J Cell Biol. 1997 Dec 29;139(7):1861-72. doi: 10.1083/jcb.139.7.1861. J Cell Biol. 1997. PMID: 9412478 Free PMC article. - Expression of stromelysin-1 and TIMP-1 in the involuting mammary gland and in early invasive tumors of the mouse.
Li F, Strange R, Friis RR, Djonov V, Altermatt HJ, Saurer S, Niemann H, Andres AC. Li F, et al. Int J Cancer. 1994 Nov 15;59(4):560-8. doi: 10.1002/ijc.2910590421. Int J Cancer. 1994. PMID: 7960227 - The role of stromelysin-1 in stromal-epithelial interactions and cancer.
Wiesen JF, Werb Z. Wiesen JF, et al. Enzyme Protein. 1996;49(1-3):174-81. doi: 10.1159/000468624. Enzyme Protein. 1996. PMID: 8797005 Review.
Cited by
- Pre-EMTing metastasis? Recapitulation of morphogenetic processes in cancer.
Berx G, Raspé E, Christofori G, Thiery JP, Sleeman JP. Berx G, et al. Clin Exp Metastasis. 2007;24(8):587-97. doi: 10.1007/s10585-007-9114-6. Epub 2007 Nov 3. Clin Exp Metastasis. 2007. PMID: 17978854 Review. - Wnt signaling activation and mammary gland hyperplasia in MMTV-LRP6 transgenic mice: implication for breast cancer tumorigenesis.
Zhang J, Li Y, Liu Q, Lu W, Bu G. Zhang J, et al. Oncogene. 2010 Jan 28;29(4):539-49. doi: 10.1038/onc.2009.339. Epub 2009 Nov 2. Oncogene. 2010. PMID: 19881541 Free PMC article. - Helicobacter pylori CagA induces a transition from polarized to invasive phenotypes in MDCK cells.
Bagnoli F, Buti L, Tompkins L, Covacci A, Amieva MR. Bagnoli F, et al. Proc Natl Acad Sci U S A. 2005 Nov 8;102(45):16339-44. doi: 10.1073/pnas.0502598102. Epub 2005 Oct 28. Proc Natl Acad Sci U S A. 2005. PMID: 16258069 Free PMC article. - Emerging functions of C/EBPβ in breast cancer.
Matherne MG, Phillips ES, Embrey SJ, Burke CM, Machado HL. Matherne MG, et al. Front Oncol. 2023 Jan 25;13:1111522. doi: 10.3389/fonc.2023.1111522. eCollection 2023. Front Oncol. 2023. PMID: 36761942 Free PMC article. Review. - Synthesis and evaluation of novel heterocyclic MMP inhibitors.
Hayashi R, Jin X, Cook GR. Hayashi R, et al. Bioorg Med Chem Lett. 2007 Dec 15;17(24):6864-70. doi: 10.1016/j.bmcl.2007.10.020. Epub 2007 Oct 17. Bioorg Med Chem Lett. 2007. PMID: 18029173 Free PMC article.
References
- Adolph S, Bartram CR, Hameister H. Mapping of the oncogenes Myc, Sis, and int-1 to the distal part of mouse chromosome 15. Cytogenet. Cell Genet. 1987;44:65–68. - PubMed
- Ahmad A, Hanby A, Dublin E, Poulsom R, Smith P, Barnes D, Rubens R, Anglard P, Hart I. Stromelysin 3: an independent prognostic factor for relapse-free survival in node-positive breast cancer and demonstration of novel breast carcinoma cell expression. Am. J. Pathol. 1998;152:721–728. - PMC - PubMed
- Bachman KE, Herman JG, Corn PG, Merlo A, Costello JF, Cavenee WK, Baylin SB, Graff JR. Methylation-associated silencing of the tissue inhibitor of metalloproteinase-3 gene suggests a suppressor role in kidney, brain, and other human cancers. Cancer Res. 1999;59:798–802. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 CA064786/CA/NCI NIH HHS/United States
- P01 CA072006-020003/CA/NCI NIH HHS/United States
- CA72006/CA/NCI NIH HHS/United States
- R01 CA057621/CA/NCI NIH HHS/United States
- CA64786/CA/NCI NIH HHS/United States
- P01 CA072006/CA/NCI NIH HHS/United States
- CA57621/CA/NCI NIH HHS/United States
- R01 CA064786-04A1/CA/NCI NIH HHS/United States
- R01 CA057621-07/CA/NCI NIH HHS/United States
- R01 CA064786/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous