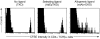CD4+ T cell division in irradiated mice requires peptides distinct from those responsible for thymic selection - PubMed (original) (raw)
CD4+ T cell division in irradiated mice requires peptides distinct from those responsible for thymic selection
J Bender et al. J Exp Med. 1999.
Abstract
We investigated the mechanism by which alpha/beta T cells expand upon transfer to T cell-deficient host mice by injecting carboxyfluorescein diacetate succinimidyl ester-labeled T cells into mice depleted of T cells by sublethal irradiation. We found that CD4+ T cells divided when transferred to irradiated hosts and that the division of more than half of these cells required class II expression. However, division of transferred CD4+ T cells did not occur in irradiated hosts that expressed class II molecules occupied solely by the peptide responsible for thymic selection, indicating that peptides distinct from those involved in thymic selection cause the division of CD4+ T cells in irradiated mice. These data establish that class II-bound peptides control the expansion of CD4+ T cells transferred to T cell-deficient hosts and suggest that the same peptides contribute to the maintenance of T cell numbers in normal mice.
Figures
Figure 1
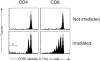
CD4+ T cells divide after transfer to irradiated hosts. Purified, CFSE-labeled B6.PL (Thy1.1) T cells were transferred to normal or 450-rad irradiated B6 (Thy1.2) mice. Each host received 1.8 × 106 B6.PL CD4+ T cells. 7 d later lymph node and spleen cells were stained with PE anti-Th1.1, CyC anti-CD8, and allophycoerythrin anti-CD4. Histograms show the intensity of CFSE fluorescence of CD4+Thy1.1+ or CD8+Thy1.1+ cells. Total CD4+Thy1.1+ cells recovered from the spleen and lymph nodes of each nonirradiated or irradiated host were 2.4 × 105 and 2.7 × 105, respectively.
Figure 2
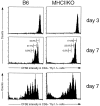
CD4+ T cells divide rapidly after transfer to untreated TCR-βKO (left) or RAG2KO (right) mice. Sublethally irradiated TCR-βKO or RAG2KO mice were injected with 3 × 106 CFSE-labeled B6.PL (left) or BALB/c (right) T cells. 7 d later lymph node and spleen cells were removed for analysis. Cells from TCR-βKO recipients were stained with PE anti-Th1.1, CyC anti-CD8, and allophycoerythrin anti-CD4, and cells from RAG2KO recipients were stained with PE anti-CD4 and CyC anti–TCR-β. The data shown represent CD4+Thy1.1+ lymph node cells from TCR-βKO recipients and CD4+TCR-β1 lymph node cells from RAG2KO recipients.
Figure 5
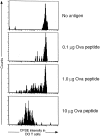
Transgenic DO T cells do not divide in irradiated mice that support thymic selection of the DO TCR. DO TCR+RAGKO BALB/c, CFSE-labeled cells were transferred to sublethally irradiated BALB/c mice. 24 h later, mice were injected intravenously with soluble OVA peptide as indicated on the figure. 7 d later, cells were stained with PE anti-CD4 and CyC KJ1 (anti-DO idiotype) and analyzed. Data shown are CD4+KJ1+ lymph node cells.
Figure 3
Much of the proliferation of CD4+ T cells in irradiated hosts requires MHC class II expression. 5 × 106 B6.PL CFSE-labeled T cells (2.1 × 106 CD4+ T cells) were transferred to 450-rad sublethally irradiated B6 or MHCIIKO mice. 7 d later lymph node and spleen cells were stained and analyzed as described in Fig. 1. Numbers indicate the percentages of cells that have not divided, divided once, or divided twice, with the percentage calculation excluding cells that have gone through more than two divisions (see Fig. 1). Total CD4+Thy1.1 cells recovered from each B6 or MHCIIKO mouse were 2.9 × 105 and 5.4 × 105, respectively.
Figure 4
The selecting ligand does not drive CD4+ T cell division in irradiated hosts. 3.8 × 105 CFSE-labeled CD4+ AbEpTKO T cells were transferred to 450-rad sublethally irradiated hosts. Hosts were: TKO (β2M KO, wtAbKO, IiKO); AbEpTKO (β2M KO, wtAbKO, IiKO, AbEp+); wtAb+DKO (β2M KO, wtAb++, IiKO). 7 d after transfer cells were stained with PE anti-CD4 and CyC anti–TCR-β and analyzed. Histograms represent the data from live CD4+TCR+ lymph node cells.
Similar articles
- Altered selection of CD4+ T cells by class II MHC bound with dominant and low abundance self-peptides.
Gaszewska-Mastalarz A, Muranski P, Chmielowski B, Kraj P, Ignatowicz L. Gaszewska-Mastalarz A, et al. J Immunol. 2000 Dec 1;165(11):6099-106. doi: 10.4049/jimmunol.165.11.6099. J Immunol. 2000. PMID: 11086042 - Mature CD4+ T cells perceive a positively selecting class II MHC/peptide complex in the periphery.
Muranski P, Chmielowski B, Ignatowicz L. Muranski P, et al. J Immunol. 2000 Mar 15;164(6):3087-94. doi: 10.4049/jimmunol.164.6.3087. J Immunol. 2000. PMID: 10706698 - Survival and homeostatic proliferation of naive peripheral CD4+ T cells in the absence of self peptide:MHC complexes.
Clarke SR, Rudensky AY. Clarke SR, et al. J Immunol. 2000 Sep 1;165(5):2458-64. doi: 10.4049/jimmunol.165.5.2458. J Immunol. 2000. PMID: 10946271 - On the self-referential nature of naive MHC class II-restricted T cells.
Viret C, He X, Janeway CA Jr. Viret C, et al. J Immunol. 2000 Dec 1;165(11):6183-92. doi: 10.4049/jimmunol.165.11.6183. J Immunol. 2000. PMID: 11086052 - Thymic selection by a single MHC/peptide ligand produces a semidiverse repertoire of CD4+ T cells.
Surh CD, Lee DS, Fung-Leung WP, Karlsson L, Sprent J. Surh CD, et al. Immunity. 1997 Aug;7(2):209-19. doi: 10.1016/s1074-7613(00)80524-5. Immunity. 1997. PMID: 9285406
Cited by
- IL-7 is critical for homeostatic proliferation and survival of naive T cells.
Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. Tan JT, et al. Proc Natl Acad Sci U S A. 2001 Jul 17;98(15):8732-7. doi: 10.1073/pnas.161126098. Epub 2001 Jul 10. Proc Natl Acad Sci U S A. 2001. PMID: 11447288 Free PMC article. - Fas (CD95/APO-1) limits the expansion of T lymphocytes in an environment of limited T-cell antigen receptor/MHC contacts.
Fortner KA, Lees RK, MacDonald HR, Budd RC. Fortner KA, et al. Int Immunol. 2011 Feb;23(2):75-88. doi: 10.1093/intimm/dxq466. Epub 2011 Jan 25. Int Immunol. 2011. PMID: 21266499 Free PMC article. - Derivation and maintenance of virtual memory CD8 T cells.
Akue AD, Lee JY, Jameson SC. Akue AD, et al. J Immunol. 2012 Mar 15;188(6):2516-23. doi: 10.4049/jimmunol.1102213. Epub 2012 Feb 3. J Immunol. 2012. PMID: 22308307 Free PMC article. - Relative Frequencies of Alloantigen-Specific Helper CD4 T Cells and B Cells Determine Mode of Antibody-Mediated Allograft Rejection.
Alsughayyir J, Chhabra M, Qureshi MS, Mallik M, Ali JM, Gamper I, Moseley EL, Peacock S, Kosmoliaptsis V, Goddard MJ, Linterman MA, Motallebzadeh R, Pettigrew GJ. Alsughayyir J, et al. Front Immunol. 2019 Jan 22;9:3039. doi: 10.3389/fimmu.2018.03039. eCollection 2018. Front Immunol. 2019. PMID: 30740108 Free PMC article. - Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells.
Cho BK, Rao VP, Ge Q, Eisen HN, Chen J. Cho BK, et al. J Exp Med. 2000 Aug 21;192(4):549-56. doi: 10.1084/jem.192.4.549. J Exp Med. 2000. PMID: 10952724 Free PMC article.
References
- Miller J.F., Osoba D. Current concepts of the immunological function of the thymus. Physiol. Rev. 1967;47:437–520. - PubMed
- Nikolic-Zugic J., Bevan M.J. Role of self-peptides in positively selecting the T-cell repertoire. Nature. 1990;344:65–67. - PubMed
- Miyazaki T., Wolf P., Tourne S., Waltzinger C., Dierich A., Barois N., Ploegh H., Benoist C., Mathis D. Mice lacking H2-M complexes, enigmatic elements of the MHC class II peptide-loading pathway. Cell. 1996;84:531–541. - PubMed
- Martin W.D., Hicks G.G., Mendiratta S.K., Leva H.I., Ruley H.E., Van Kaer L. H2-M mutant mice are defective in the peptide loading of class II molecules, antigen presentation, and T cell repertoire selection. Cell. 1996;84:543–550. - PubMed
- Ignatowicz L., Kappler J., Marrack P. The repertoire of T cells shaped by a single MHC/peptide ligand. Cell. 1996;84:521–529. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI018785/AI/NIAID NIH HHS/United States
- AI22295/AI/NIAID NIH HHS/United States
- AI18785/AI/NIAID NIH HHS/United States
- R01 AI017134/AI/NIAID NIH HHS/United States
- R37 AI018785/AI/NIAID NIH HHS/United States
- R56 AI018785/AI/NIAID NIH HHS/United States
- P01 AI022295/AI/NIAID NIH HHS/United States
- R56 AI017134/AI/NIAID NIH HHS/United States
- AI17134/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials