UmuD'(2)C is an error-prone DNA polymerase, Escherichia coli pol V - PubMed (original) (raw)
UmuD'(2)C is an error-prone DNA polymerase, Escherichia coli pol V
M Tang et al. Proc Natl Acad Sci U S A. 1999.
Abstract
The damage-inducible UmuD' and UmuC proteins are required for most SOS mutagenesis in Escherichia coli. Our recent assay to reconstitute this process in vitro, using a native UmuD'(2)C complex, revealed that the highly purified preparation contained DNA polymerase activity. Here we eliminate the possibility that this activity is caused by a contaminating DNA polymerase and show that it is intrinsic to UmuD'(2)C. E. coli dinB has recently been shown to have DNA polymerase activity (pol IV). We suggest that UmuD'(2)C, the fifth DNA polymerase discovered in E. coli, be designated as E. coli pol V. In the presence of RecA, beta sliding clamp, gamma clamp loading complex, and E. coli single-stranded binding protein (SSB), pol V's polymerase activity is highly "error prone" at both damaged and undamaged DNA template sites, catalyzing efficient bypass of abasic lesions that would otherwise severely inhibit replication by pol III holoenzyme complex (HE). Pol V bypasses a site-directed abasic lesion with an efficiency about 100- to 150-fold higher than pol III HE. In accordance with the "A-rule," dAMP is preferentially incorporated opposite the lesion. A pol V mutant, UmuD'(2)C104 (D101N), has no measurable lesion bypass activity. A kinetic analysis shows that addition of increasing amounts of pol III to a fixed level of pol V inhibits lesion bypass, demonstrating that both enzymes compete for free 3'-OH template-primer ends. We show, however, that despite competition for primer-3'-ends, pol V and pol III HE can nevertheless interact synergistically to stimulate synthesis downstream from a template lesion.
Figures
Figure 1
Separation of pol IIIts from the UmuD′2C complex by using Superdex 200 gel filtration. (Upper) Superdex 200 fractions (30 μl) containing pol III α subunit [designated as α (Left)] were resolved on an 8% SDS/PAGE gel and visualized by chemiluminescent immunodetection by using antiserum directed against α subunit. (Lower) Superdex 200 fractions (20 μl) containing UmuC protein [designated as C (Left)] were visualized on a 12% SDS/PAGE gel stained with Coomassie blue R-250. The presence of UmuC and UmuD proteins was verified by using UmuC and UmuD′ antisera (data not shown).
Figure 2
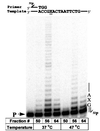
UmuD′2C (E. coli polV) has DNA polymerase activity. UmuD′2C was purified from a ΔpolB dnaE1026ts strain, RW588. Sephadex 200 fractions fx (50) containing predominantly pol IIIts; fx (56) containing pol IIIts + UmuD′2C and fx (64) containing UmuD′2C having no detectable pol IIIts were assayed for polymerase activity by extension of a 32P-labeled 30-mer primer annealed to a linear M13 DNA template, at permissive (37°C) and nonpermissive (47°C) temperatures. A single running-start base, C, is incorporated opposite G to reach the abasic lesion, X. The left-hand lane contains the primer (P) in the absence of proteins. Each reaction mixture contains 1.5 μl of the indicated fraction from a Superdex 200 gel filtration column (Fig. 1). All reactions were carried out in the presence of pol I antibody, RecA, SSB, β, γ complex, 4 dNTPs, and ATP.
Figure 3
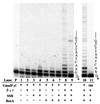
Protein cofactor requirements for UmuD′2C (E. coli pol V)-catalyzed lesion bypass. Each reaction in lanes 1–9 contains 1.5 μl of Superdex 200 fx 64 containing UmuD′2C (having no detectable pol IIIts), pol I antibody, and various combinations of RecA, β, γ complex, and SSB, as indicated (lanes 1–9). Running-start reactions, in which C is incorporated opposite template G to reach the abasic site, were carried out at 37°C, with all four dNTPs present in lanes 1 to 8, but with dCTP omitted in lane 9. Reactions were run in the presence of 5% polyethylene glycol. A portion of the template sequence is shown at the right of lane 9, where X represents an abasic site. The left-hand lane contains the primer (P) in the absence of proteins. Standing-start reactions, in which the first incorporated nucleotide occurs opposite X, were run for wild-type UmuD′2C (lane 10) and for the mutant UmuD′2C104 (D101N) (lane 11), each at a concentration of 200 nM. A portion of the template, shown at the right of lane 11, has the same sequence as the running-start template, but uses a primer terminating one base before the lesion. The asterisk (*) designates a 32P-label at the 5′-primer terminus. The running-start primer-template DNA is shown at the top of Fig. 2.
Figure 4
Inhibition of UmuD′2C (E. coli pol V)-catalyzed translesion synthesis by wild-type pol III. A running-start C (20 μM dCTP) is incorporated opposite template G before reaching the abasic lesion, X. The concentration of dATP used for incorporation opposite X was varied to measure the kinetics of incorporation by either UmuD′2C, wild-type pol III, or a combination of both proteins. The UmuD′2C complex used in the reactions was purified from a strain containing wild-type pol III (18). Michaelis–Menten saturation plots of the translesion synthesis rate vs. dATP concentration are shown at the right. The translesion synthesis rate V is obtained by measuring IXΣ/IX−1 as a function of dATP concentration, where IXΣ are the integrated gel band intensities for incorporation at the site of the lesion and beyond, and IX−1 is the integrated gel band intensity at the G site, before reaching the lesion (see Materials and Methods). (A) Translesion synthesis catalyzed by UmuD′2C, pol III core HE, or combinations of both. (B) Translesion synthesis catalyzed by UmuD′2C, pol III α HE, or combinations of both. The dATP concentrations used were 0, 3, 10, 30, 100, 300, and 1,000 μM for A and 0, 2, 10, 50, 200, 500, and 1,000 μM for B. UmuD′2C is present at 200 nM in each experiment. The reactions were run at 37°C in the presence of RecA, SSB, and β, γ complex.
Figure 5
Kinetic analysis of the effect of wild-type pol III on UmuD′2C (E. coli pol V)-catalyzed translesion synthesis**.** Lineweaver–Burk double reciprocal plots for pol III HE and α HE (Upper) were generated from the kinetic data given in Fig. 4. Apparent _K_m and _V_max values and their ratios characterizing translesion synthesis by UmuD′2C (designated as Umu) in the absence and presence of pol III are provided in the tables below.
Similar articles
- Activity of the purified mutagenesis proteins UmuC, UmuD', and RecA in replicative bypass of an abasic DNA lesion by DNA polymerase III.
Rajagopalan M, Lu C, Woodgate R, O'Donnell M, Goodman MF, Echols H. Rajagopalan M, et al. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10777-81. doi: 10.1073/pnas.89.22.10777. Proc Natl Acad Sci U S A. 1992. PMID: 1438275 Free PMC article. - Visualization of two binding sites for the Escherichia coli UmuD'(2)C complex (DNA pol V) on RecA-ssDNA filaments.
Frank EG, Cheng N, Do CC, Cerritelli ME, Bruck I, Goodman MF, Egelman EH, Woodgate R, Steven AC. Frank EG, et al. J Mol Biol. 2000 Mar 31;297(3):585-97. doi: 10.1006/jmbi.2000.3591. J Mol Biol. 2000. PMID: 10731413 - The mutagenesis protein UmuC is a DNA polymerase activated by UmuD', RecA, and SSB and is specialized for translesion replication.
Reuven NB, Arad G, Maor-Shoshani A, Livneh Z. Reuven NB, et al. J Biol Chem. 1999 Nov 5;274(45):31763-6. doi: 10.1074/jbc.274.45.31763. J Biol Chem. 1999. PMID: 10542196 - Roles of DNA polymerases V and II in SOS-induced error-prone and error-free repair in Escherichia coli.
Pham P, Rangarajan S, Woodgate R, Goodman MF. Pham P, et al. Proc Natl Acad Sci U S A. 2001 Jul 17;98(15):8350-4. doi: 10.1073/pnas.111007198. Proc Natl Acad Sci U S A. 2001. PMID: 11459974 Free PMC article. Review. - The "tale" of UmuD and its role in SOS mutagenesis.
Gonzalez M, Woodgate R. Gonzalez M, et al. Bioessays. 2002 Feb;24(2):141-8. doi: 10.1002/bies.10040. Bioessays. 2002. PMID: 11835278 Review.
Cited by
- Diverse responses to UV light exposure in Acinetobacter include the capacity for DNA damage-induced mutagenesis in the opportunistic pathogens Acinetobacter baumannii and Acinetobacter ursingii.
Hare JM, Bradley JA, Lin CL, Elam TJ. Hare JM, et al. Microbiology (Reading). 2012 Mar;158(Pt 3):601-611. doi: 10.1099/mic.0.054668-0. Epub 2011 Nov 24. Microbiology (Reading). 2012. PMID: 22117008 Free PMC article. - Bridging the gap: a family of novel DNA polymerases that replicate faulty DNA.
Johnson RE, Washington MT, Prakash S, Prakash L. Johnson RE, et al. Proc Natl Acad Sci U S A. 1999 Oct 26;96(22):12224-6. doi: 10.1073/pnas.96.22.12224. Proc Natl Acad Sci U S A. 1999. PMID: 10535901 Free PMC article. No abstract available. - Error-free and error-prone lesion bypass by human DNA polymerase kappa in vitro.
Zhang Y, Yuan F, Wu X, Wang M, Rechkoblit O, Taylor JS, Geacintov NE, Wang Z. Zhang Y, et al. Nucleic Acids Res. 2000 Nov 1;28(21):4138-46. doi: 10.1093/nar/28.21.4138. Nucleic Acids Res. 2000. PMID: 11058110 Free PMC article. - Manganese Is Required for the Rapid Recovery of DNA Synthesis following Oxidative Challenge in Escherichia coli.
Hutfilz CR, Wang NE, Hoff CA, Lee JA, Hackert BJ, Courcelle J, Courcelle CT. Hutfilz CR, et al. J Bacteriol. 2019 Nov 20;201(24):e00426-19. doi: 10.1128/JB.00426-19. Print 2019 Dec 15. J Bacteriol. 2019. PMID: 31570529 Free PMC article.
References
- Friedberg E C, Walker G C, Siede W. DNA Repair and Mutagenesis. Washington, DC: ASM Press; 1995.
- Koch W H, Woodgate R. In: The SOS Response. Nickoloff J A, Hoekstra M F, editors. Totowa, NJ: Humana; 1988. pp. 107–134.
- Sommer S, Knezevic J, Bailone A, Devoret R. Mol Gen Genet. 1993;239:137–144. - PubMed
- Kato T, Shinoura Y. Mol Gen Genet. 1977;156:121–131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases