NF-kappaB-mediated up-regulation of Bcl-x and Bfl-1/A1 is required for CD40 survival signaling in B lymphocytes - PubMed (original) (raw)
NF-kappaB-mediated up-regulation of Bcl-x and Bfl-1/A1 is required for CD40 survival signaling in B lymphocytes
H H Lee et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Activation of CD40 is essential for thymus-dependent humoral immune responses and rescuing B cells from apoptosis. Many of the effects of CD40 are believed to be achieved through altered gene expression. In addition to Bcl-x, a known CD40-regulated antiapoptotic molecule, we identified a related antiapoptotic molecule, A1/Bfl-1, as a CD40-inducible gene. Inhibition of the NF-kappaB pathway by overexpression of a dominant-active inhibitor of NF-kappaB abolished CD40-induced up-regulation of both the Bfl-1 and Bcl-x genes and also eliminated the ability of CD40 to rescue Fas-induced cell death. Within the upstream promoter region of Bcl-x, a potential NF-kappaB-binding sequence was found to support NF-kappaB-dependent transcriptional activation. Furthermore, expression of physiological levels of Bcl-x protected B cells from Fas-mediated apoptosis in the absence of NF-kappaB signaling. Thus, our results suggest that CD40-mediated cell survival proceeds through NF-kappaB-dependent up-regulation of Bcl-2 family members.
Figures
Figure 1
Bcl-x and Bfl-1 are rapidly induced primary response genes. (A) CD40 inducibility of Bfl-1 was confirmed in three human B cell lines cultured for 16 hr in the presence or absence of G28.5. All cells were treated with 1 μg/ml G28.5 at a density of 1 × 106 cells per ml. Eighteen micrograms of total RNA was loaded per lane, and the Northern blot was hybridized with a human Bfl-1 probe. The Northern blot was stripped, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression was probed as a standard. (B) The kinetics of Bfl-1 and Bcl-x induction in Daudi cells were determined by examining gene expression after various periods of G28.5 stimulation. To determine the requirement for new protein synthesis in the CD40-mediated induction of these genes, cells were pretreated for 30 min with the protein synthesis inhibitor cycloheximide (CHX) at 25 μg/ml and then stimulated with G28.5 antibody for various periods of time in the presence of CHX. All cells were treated with 1 μg/ml G28.5 at a density of 1 × 106 cells per ml. Eighteen micrograms of total RNA was loaded per lane, and the Northern blots were hybridized with a human Bfl-1 or Bcl-x probe. Northern blots were stripped, and GAPDH expression was probed as a standard.
Figure 2
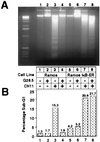
Cells expressing IκB-ER have a functional block in NF-κB signaling and do not up-regulate Bcl-x and Bfl-1 in response to CD40 activation. (A) Cells were stimulated for 25 min in the presence or absence of 1 μg/ml G28.5 antibody, and the nuclear extracts were then processed as described previously. Six micrograms of nuclear extracts then were used in binding assays with the radiolabeled MHC II oligonucleotide. Cells induced with 4-OHT were pretreated with 200 nM of the synthetic estrogen for 3 hr before G28.5-mediated activation. (B) Daudi, Daudi-IκB-ER, Ramos, and Ramos-IκB-ER cells were pretreated with 4-OHT for 45 min and then cultured for 8 hr in 4-OHT alone or 4-OHT plus G28.5. Eighteen micrograms of total RNA was loaded per lane, and the Northern blots were hybridized with a human Bfl-1 or Bcl-x probe. Northern blots were stripped and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression was probed as a standard. (C) Daudi, Daudi-IκB-ER, Ramos, and Ramos-IκB-ER cells were pretreated with 4-OHT for 2 hr and then stimulated with G28.5 in the presence of 4-OHT for different periods of time. Total cell extracts from 1 × 106 cells were loaded per lane, and Western blotting was performed to detect Bcl-x expression.
Figure 3
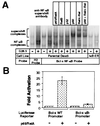
Rel members are capable of mediating transcriptional activation through the Bcl-x promoter by binding to NF-κB cis elements. (A) The NF-κB complex binding to the putative NF-κB-binding sites within the Bcl-x promoter consists predominantly of p50, p65, and c-Rel. Nuclear extract harvests and binding reactions were performed as described previously. Supershifts were performed by the addition of the appropriate antibody to the nuclear extracts on ice 20 min before the binding reaction. (B) p65 is capable of mediating transcriptional activation through the Bcl-x promoter. A Bcl-x promoter fragment spanning −640 to +9 relative to the transcriptional start site (Bcl-x WT promoter) and another fragment missing the NF-κB-binding sequence (Bcl-x κB- promoter) were cloned into the pGL2-Basic luciferase reporter vector. 293T cells were transfected with 100 ng of the indicated reporter plasmids and either 2 μg of p65/RelA expression vector or 2 μg of pBABE-puro control vector as carrier. Samples were harvested 36 hr after transfection and assessed for luciferase activity.
Figure 4
CD40-mediated rescue of Fas-induced apoptosis is ablated in the absence of NF-κB signaling. Cells (6 × 106) in 5 ml of medium were either untreated or treated for 24 hr with 1 μg/ml G28.5, 500 ng/ml CH11 Fas-activating antibody, or both. Cells were harvested, and DNA fragmentation (A) and the sub-G1 fraction (B) were assayed as described in Materials and Methods.
Figure 5
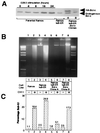
Expression of exogenous Bcl-x protects against Fas-mediated cell death. (A) Ramos IκB-ER cells were transfected by electroporation with a pEBB-puro-Bcl-x-HA construct, and stable clones were isolated by limiting dilution plating and selection with puromycin. Expression of exogenous Bcl-x then was assessed by Western analysis and compared with the levels of endogenous Bcl-x that are up-regulated in response to CD40 activation by the G28.5 antibody. (B) DNA fragmentation was assessed in parental Ramos, Ramos IκB-ER, and Ramos IκB-ER-Bcl-x clones A and B after being untreated or treated for 24 hr with CH11. (C) The sub-G1 fraction was assessed by FACScan analysis after propidium iodide staining in Ramos cells, Ramos IκB-ER cells, and Ramos IκB-ER-Bcl-x clones A and B after being untreated or treated for 24 hr with the CH11 antibody.
Similar articles
- Nuclear factor kappa B-dependent activation of the antiapoptotic bfl-1 gene by the Epstein-Barr virus latent membrane protein 1 and activated CD40 receptor.
D'Souza BN, Edelstein LC, Pegman PM, Smith SM, Loughran ST, Clarke A, Mehl A, Rowe M, Gélinas C, Walls D. D'Souza BN, et al. J Virol. 2004 Feb;78(4):1800-16. doi: 10.1128/jvi.78.4.1800-1816.2004. J Virol. 2004. PMID: 14747545 Free PMC article. - Phosphatidylinositol 3-kinase and NF-kappa B/Rel are at the divergence of CD40-mediated proliferation and survival pathways.
Andjelic S, Hsia C, Suzuki H, Kadowaki T, Koyasu S, Liou HC. Andjelic S, et al. J Immunol. 2000 Oct 1;165(7):3860-7. doi: 10.4049/jimmunol.165.7.3860. J Immunol. 2000. PMID: 11034392 - The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-kappaB that blocks TNFalpha-induced apoptosis.
Zong WX, Edelstein LC, Chen C, Bash J, Gélinas C. Zong WX, et al. Genes Dev. 1999 Feb 15;13(4):382-7. doi: 10.1101/gad.13.4.382. Genes Dev. 1999. PMID: 10049353 Free PMC article. - Inducible resistance to Fas-mediated apoptosis in B cells.
Rothstein TL. Rothstein TL. Cell Res. 2000 Dec;10(4):245-66. doi: 10.1038/sj.cr.7290053. Cell Res. 2000. PMID: 11191348 Review. - Transcriptional regulation of the BCL-X gene by NF-kappaB is an element of hypoxic responses in the rat brain.
Glasgow JN, Qiu J, Rassin D, Grafe M, Wood T, Perez-Pol JR. Glasgow JN, et al. Neurochem Res. 2001 Jun;26(6):647-59. doi: 10.1023/a:1010987220034. Neurochem Res. 2001. PMID: 11519724 Review.
Cited by
- CD40 expression and its prognostic significance in human gastric carcinoma.
Guo J, Xiao JJ, Zhang X, Fan KX. Guo J, et al. Med Oncol. 2015 Mar;32(3):63. doi: 10.1007/s12032-014-0463-0. Epub 2015 Feb 10. Med Oncol. 2015. PMID: 25665853 - MCL-1 is required throughout B-cell development and its loss sensitizes specific B-cell subsets to inhibition of BCL-2 or BCL-XL.
Vikström IB, Slomp A, Carrington EM, Moesbergen LM, Chang C, Kelly GL, Glaser SP, Jansen JH, Leusen JH, Strasser A, Huang DC, Lew AM, Peperzak V, Tarlinton DM. Vikström IB, et al. Cell Death Dis. 2016 Aug 25;7(8):e2345. doi: 10.1038/cddis.2016.237. Cell Death Dis. 2016. PMID: 27560714 Free PMC article. - Role of immune escape mechanisms in Hodgkin's lymphoma development and progression: a whole new world with therapeutic implications.
de la Cruz-Merino L, Lejeune M, Nogales Fernández E, Henao Carrasco F, Grueso López A, Illescas Vacas A, Pulla MP, Callau C, Álvaro T. de la Cruz-Merino L, et al. Clin Dev Immunol. 2012;2012:756353. doi: 10.1155/2012/756353. Epub 2012 Aug 15. Clin Dev Immunol. 2012. PMID: 22927872 Free PMC article. Review. - Rotavirus infection alters peripheral T-cell homeostasis in children with acute diarrhea.
Wang Y, Dennehy PH, Keyserling HL, Tang K, Gentsch JR, Glass RI, Jiang B. Wang Y, et al. J Virol. 2007 Apr;81(8):3904-12. doi: 10.1128/JVI.01887-06. Epub 2007 Jan 31. J Virol. 2007. PMID: 17267507 Free PMC article. - One path to cell death in the nervous system.
Glasgow J, Perez-Polo R. Glasgow J, et al. Neurochem Res. 2000 Oct;25(9-10):1373-83. doi: 10.1023/a:1007612716591. Neurochem Res. 2000. PMID: 11059808 Review.
References
- Smith C A, Farrah T, Goodwin R G. Cell. 1994;76:959–962. - PubMed
- Callard R, Armitage R, Fanslow W, Spriggs M. Immunol Today. 1993;14:559–564. - PubMed
- Durie F H, Foy T M, Masters S R, Laman J D, Noelle R J. Immunol Today. 1994;15:406–411. - PubMed
- Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Sumatsu S, Yoshida N, Kishimoto T, Kikutani H. Immunity. 1994;1:167–178. - PubMed
- Xu J, Foy T M, Lsaman J D, Elliott E A, Dunn J J, Waldschmidt T J, Elsemore J, Noelle R J, Flavell R A. Immunity. 1994;1:423–431. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous