Mitotic recombination produces the majority of recessive fibroblast variants in heterozygous mice - PubMed (original) (raw)
Mitotic recombination produces the majority of recessive fibroblast variants in heterozygous mice
C Shao et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Mice heterozygous at Aprt (adenine phosphoribosyltransferase) were used as a model to study in vivo loss of heterozygosity (LOH) in normal fibroblasts. Somatic cell variants that exhibited functional loss of the wild-type Aprt in vivo were recovered as APRT-deficient cell colonies after culturing in selection medium containing 2, 6-diaminopurine (DAP), an adenine analog that is toxic only to cells with APRT enzyme activity. DAP-resistant (DAP(r)) fibroblast variants were recovered at a median frequency of 12 x 10(-5) from individual ears from progeny of crosses between mouse strains 129/Sv and C3H/HeJ. The frequency of DAP(r) variants varied greatly among individual ears, suggesting that they preexisted in vivo and arose at various times during development. Polymorphic molecular markers and a cytological marker on the centromere of chromosome 8 made it possible to discriminate between each of six possible mechanistic pathways of LOH. The majority (about 80%) of the DAP(r) variants were a consequence of mitotic recombination. The prevalence of mitotic recombination in regions proximal to Aprt did not correlate with meiotic map distances. In particular, there was a higher than expected frequency of crossovers within the interval 59 cM to 67 cM. The high spontaneous frequency of Aprt LOH, mediated primarily by mitotic recombination, is fully consistent with our previous results with human peripheral T cells from individuals known to be heterozygous at APRT. Thus, this Aprt heterozygote mouse is a valid model for studying somatic mutagenesis and mitotic recombination in vivo.
Figures
Figure 1
Allele-specific PCR of representative DAPr clones. Clones 2, 3, 4, 6, 7, 9, 10, 11, and 12, designated as class I, exhibited physical loss of Aprt+. Clones 1, 5, and 8, designated as class II, retained Aprt+.
Figure 2
Intervals of LOH in class I clones. The lines correspond to the interval for which the SSR markers remained heterozygous. All markers right to the lines exhibited LOH. The number of independent events in each group is shown on the left. The map positions of the SSR markers are according to Mouse Genome Database [
http://www.informatics.jax.org
(6/98)].
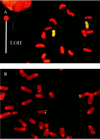
Figure 3
Cytogenetic evidence that LOH was caused by mitotic recombination. (A) Whole-chromosome painting of a class I DAPr fibroblast clone. Chromosome regions exhibiting hybridization signal are green, otherwise they stain red. Chromosome 8 of strain C3H/HeJ (Chr8C3H) exhibits a large centromeric region. Although SSR genotyping showed a large interval of LOH (loss of C3H alleles), the homologue with a large centromere, Chr8C3H, exhibited no corresponding terminal deletion. (B) Aprt FISH of a class I DAPr clone. SSR genotyping showed terminal LOH (loss of C3H alleles) beginning at 59 cM, but Aprt hybridization signals were evident on both Chr8129 and Chr8C3H.
Similar articles
- In vivo loss of heterozygosity in T-cells of B6C3F1 Aprt(+/-) mice.
Liang L, Deng L, Shao C, Stambrook PJ, Tischfield JA. Liang L, et al. Environ Mol Mutagen. 2000;35(2):150-7. Environ Mol Mutagen. 2000. PMID: 10712749 - APRT: a versatile in vivo resident reporter of local mutation and loss of heterozygosity.
Stambrook PJ, Shao C, Stockelman M, Boivin G, Engle SJ, Tischfield JA. Stambrook PJ, et al. Environ Mol Mutagen. 1996;28(4):471-82. doi: 10.1002/(SICI)1098-2280(1996)28:4<471::AID-EM25>3.0.CO;2-B. Environ Mol Mutagen. 1996. PMID: 8991080 - High frequency in vivo loss of heterozygosity is primarily a consequence of mitotic recombination.
Gupta PK, Sahota A, Boyadjiev SA, Bye S, Shao C, O'Neill JP, Hunter TC, Albertini RJ, Stambrook PJ, Tischfield JA. Gupta PK, et al. Cancer Res. 1997 Mar 15;57(6):1188-93. Cancer Res. 1997. PMID: 9067291 - [Adenine phosphoribosyltransferase (APRT)].
Kamatani N. Kamatani N. Nihon Rinsho. 1996 Dec;54(12):3213-9. Nihon Rinsho. 1996. PMID: 8976094 Review. Japanese. - Germline and somatic mutation at the APRT locus of mice and man.
Tischfield JA, Engle SJ, Gupta PK, Bye S, Boyadjiev S, Shao C, O'Neill P, Albertini RJ, Stambrook PJ, Sahota AS. Tischfield JA, et al. Adv Exp Med Biol. 1994;370:661-4. doi: 10.1007/978-1-4615-2584-4_137. Adv Exp Med Biol. 1994. PMID: 7660991 Review. No abstract available.
Cited by
- Mitotic Antipairing of Homologous Chromosomes.
Hua LL, Casas CJ, Mikawa T. Hua LL, et al. Results Probl Cell Differ. 2022;70:191-220. doi: 10.1007/978-3-031-06573-6_6. Results Probl Cell Differ. 2022. PMID: 36348108 Free PMC article. - Speciation of Genes and Genomes: Conservation of DNA Polymorphism by Barriers to Recombination Raised by Mismatch Repair System.
Radman M. Radman M. Front Genet. 2022 Feb 28;13:803690. doi: 10.3389/fgene.2022.803690. eCollection 2022. Front Genet. 2022. PMID: 35295946 Free PMC article. Review. - An overlooked subset of Cx3cr1wt/wt microglia in the Cx3cr1CreER-Eyfp/wt mouse has a repopulation advantage over Cx3cr1CreER-Eyfp/wt microglia following microglial depletion.
Zhou K, Han J, Lund H, Boggavarapu NR, Lauschke VM, Goto S, Cheng H, Wang Y, Tachi A, Xie C, Zhu K, Sun Y, Osman AM, Liang D, Han W, Gemzell-Danielsson K, Betsholtz C, Zhang XM, Zhu C, Enge M, Joseph B, Harris RA, Blomgren K. Zhou K, et al. J Neuroinflammation. 2022 Jan 21;19(1):20. doi: 10.1186/s12974-022-02381-6. J Neuroinflammation. 2022. PMID: 35062962 Free PMC article. - Excision of mutagenic replication-blocking lesions suppresses cancer but promotes cytotoxicity and lethality in nitrosamine-exposed mice.
Kay JE, Corrigan JJ, Armijo AL, Nazari IS, Kohale IN, Torous DK, Avlasevich SL, Croy RG, Wadduwage DN, Carrasco SE, Dertinger SD, White FM, Essigmann JM, Samson LD, Engelward BP. Kay JE, et al. Cell Rep. 2021 Mar 16;34(11):108864. doi: 10.1016/j.celrep.2021.108864. Cell Rep. 2021. PMID: 33730582 Free PMC article. - Reduced C9ORF72 function exacerbates gain of toxicity from ALS/FTD-causing repeat expansion in C9orf72.
Zhu Q, Jiang J, Gendron TF, McAlonis-Downes M, Jiang L, Taylor A, Diaz Garcia S, Ghosh Dastidar S, Rodriguez MJ, King P, Zhang Y, La Spada AR, Xu H, Petrucelli L, Ravits J, Da Cruz S, Lagier-Tourenne C, Cleveland DW. Zhu Q, et al. Nat Neurosci. 2020 May;23(5):615-624. doi: 10.1038/s41593-020-0619-5. Epub 2020 Apr 13. Nat Neurosci. 2020. PMID: 32284607 Free PMC article.
References
- Malkin D, Li F P, Strong L C, Fraumeni J F, Nelson C E, Kim D H, Kassel J, Gryka M A, Bischoff F Z, Tainsky M A, et al. Science. 1990;250:1233–1238. - PubMed
- Cavenee W K, Dryja T P, Philips R A, Benedict W F, Godbout R, Gallie B L, Murphree A L, Strong L C, White R L. Nature (London) 1983;305:779–784. - PubMed
- Kinzler K W, Vogelstein B. Cell. 1996;87:159–170. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources