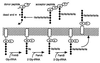The essential Staphylococcus aureus gene fmhB is involved in the first step of peptidoglycan pentaglycine interpeptide formation - PubMed (original) (raw)
The essential Staphylococcus aureus gene fmhB is involved in the first step of peptidoglycan pentaglycine interpeptide formation
S Rohrer et al. Proc Natl Acad Sci U S A. 1999.
Abstract
The factor catalyzing the first step in the synthesis of the characteristic pentaglycine interpeptide in Staphylococcus aureus peptidoglycan was found to be encoded by the essential gene fmhB. We have analyzed murein composition and structure synthesized when fmhB expression is reduced. The endogenous fmhB promoter was substituted with the xylose regulon from Staphylococcus xylosus, which allowed glucose-controlled repression of fmhB transcription. Repression of fmhB reduced growth and triggered a drastic accumulation of uncrosslinked, unmodified muropeptide monomer precursors at the expense of the oligomeric fraction, leading to a substantial decrease in overall peptidoglycan crosslinking. The composition of the predominant muropeptide was confirmed by MS to be N-acetylglucosamine-(beta-1,4)-N-acetylmuramic acid(-L-Ala-D-iGln-L-Lys-D-Ala-D-Ala), proving that FmhB is involved in the attachment of the first glycine to the pentaglycine interpeptide. This interpeptide plays an important role in crosslinking and stability of the S. aureus cell wall, acts as an anchor for cell wall-associated proteins, determinants of pathogenicity, and is essential for the expression of methicillin resistance. Any shortening of the pentaglycine side chain reduces or even abolishes methicillin resistance, as occurred with fmhB repression. Because of its key role FmhB is a potential target for novel antibacterial agents that could control the threat of emerging multiresistant S. aureus.
Figures
Figure 1
Integration of pSR1 into the S. aureus chromosome. Black: fmhB gene; hatched: xylose regulon (xylR and the operator region of xylA); white: fmhB promoter region; bold line: pOX7 plasmid DNA. Relevant restriction sites are indicated as follows: H, _Hin_dIII; B, _Bam_HI; K, _Kpn_I; E, _Eco_RI. Primers used for cloning and PCR controls are indicated by arrows and numbered as in Materials and Methods. The region spanned by the internal DNA probe used for Southern and Northern blots is indicated by a line above fmhB. Not to scale.
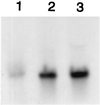
Figure 2
Northern blots of fmhB mRNA. Strain SR18 was grown in LB at 42°C to exponential phase in presence of glucose (lane 1), unsupplemented LB (lane 2), or xylose (lane 3). RNA was probed with the fmhB probe shown in Fig. 1.
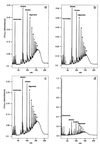
Figure 3
Muropeptide profiles. Isolated peptidoglycan was digested with muramidase and muropeptides were subjected to reversed-phase HPLC. Strain BB270 grown at 42°C (a) with xylose, (b) with glucose. Strain SR18 grown (c) with xylose, (d) with glucose. The muropeptide fractions are indicated as follows: monomers, corresponding to uncrosslinked subunits (retention time 10–45 min); dimers, corresponding to two crosslinked muropeptides (45–85 min); trimers, corresponding to three crosslinked muropeptides (85–105 min); and oligomers, corresponding to three or more crosslinked muropeptides. More highly crosslinked muropeptides in the oligomeric fraction are indicated by numbers.
Figure 4
Detailed view of the monomeric and dimeric muropeptide pattern of SR18 grown with fmhB induction (a) and repression (b). Peaks were identified by their retention time upon comparison with standard samples. Peak M1 was analyzed by MS. The peak nomenclature is shown in Table 3.
Figure 5
Pentaglycine side-chain formation and peptidoglycan crosslinking. (Upper) Transpeptidase reaction crosslinking the fifth glycine residue of the acceptor muropeptide to the
d
-Ala of the donor stem peptide with release of the terminal
d
-Ala. Direct linkage to the ɛ-amino group of lysine does not occur in S. aureus. (Lower) Synthesis of the pentaglycine side chain at the membrane-bound lipid II intermediate.
Similar articles
- epr, which encodes glycylglycine endopeptidase resistance, is homologous to femAB and affects serine content of peptidoglycan cross bridges in Staphylococcus capitis and Staphylococcus aureus.
Sugai M, Fujiwara T, Ohta K, Komatsuzawa H, Ohara M, Suginaka H. Sugai M, et al. J Bacteriol. 1997 Jul;179(13):4311-8. doi: 10.1128/jb.179.13.4311-4318.1997. J Bacteriol. 1997. PMID: 9209049 Free PMC article. - Identification of three additional femAB-like open reading frames in Staphylococcus aureus.
Tschierske M, Mori C, Rohrer S, Ehlert K, Shaw KJ, Berger-Bächi B. Tschierske M, et al. FEMS Microbiol Lett. 1999 Feb 15;171(2):97-102. doi: 10.1111/j.1574-6968.1999.tb13417.x. FEMS Microbiol Lett. 1999. PMID: 10077832 - Cell wall monoglycine cross-bridges and methicillin hypersusceptibility in a femAB null mutant of methicillin-resistant Staphylococcus aureus.
Strandén AM, Ehlert K, Labischinski H, Berger-Bächi B. Strandén AM, et al. J Bacteriol. 1997 Jan;179(1):9-16. doi: 10.1128/jb.179.1.9-16.1997. J Bacteriol. 1997. PMID: 8981974 Free PMC article. - Staphylococcus aureus as an infectious agent: overview of biochemistry and molecular genetics of its pathogenicity.
Plata K, Rosato AE, Wegrzyn G. Plata K, et al. Acta Biochim Pol. 2009;56(4):597-612. Epub 2009 Dec 11. Acta Biochim Pol. 2009. PMID: 20011685 Review. - Lysostaphin: Engineering and Potentiation toward Better Applications.
Zha J, Li J, Su Z, Akimbekov N, Wu X. Zha J, et al. J Agric Food Chem. 2022 Sep 21;70(37):11441-11457. doi: 10.1021/acs.jafc.2c03459. Epub 2022 Sep 9. J Agric Food Chem. 2022. PMID: 36082619 Review.
Cited by
- Roles of tRNA in cell wall biosynthesis.
Dare K, Ibba M. Dare K, et al. Wiley Interdiscip Rev RNA. 2012 Mar-Apr;3(2):247-64. doi: 10.1002/wrna.1108. Epub 2012 Jan 19. Wiley Interdiscip Rev RNA. 2012. PMID: 22262511 Free PMC article. Review. - Amoxicillin Administration Regimen and Resistance Mechanisms of Staphylococcus aureus Established in Tissue Cage Infection Model.
Yao Q, Gao L, Xu T, Chen Y, Yang X, Han M, He X, Li C, Zhou R, Yang Y. Yao Q, et al. Front Microbiol. 2019 Jul 22;10:1638. doi: 10.3389/fmicb.2019.01638. eCollection 2019. Front Microbiol. 2019. PMID: 31396174 Free PMC article. - Antistaphylococcal nanocomposite films based on enzyme-nanotube conjugates.
Pangule RC, Brooks SJ, Dinu CZ, Bale SS, Salmon SL, Zhu G, Metzger DW, Kane RS, Dordick JS. Pangule RC, et al. ACS Nano. 2010 Jul 27;4(7):3993-4000. doi: 10.1021/nn100932t. ACS Nano. 2010. PMID: 20604574 Free PMC article. - Comparative Transcriptome Analysis Reveals Differentially Expressed Genes Related to Antimicrobial Properties of Lysostaphin in Staphylococcus aureus.
Yan X, Xie Y, Li C, Donovan DM, Gehring A, Irwin P, He Y. Yan X, et al. Antibiotics (Basel). 2022 Jan 18;11(2):125. doi: 10.3390/antibiotics11020125. Antibiotics (Basel). 2022. PMID: 35203727 Free PMC article. - Reduced Growth of Staphylococcus aureus Under High Glucose Conditions Is Associated With Decreased Pentaglycine Expression.
Luo Z, Yue S, Chen T, She P, Wu Y, Wu Y. Luo Z, et al. Front Microbiol. 2020 Nov 2;11:537290. doi: 10.3389/fmicb.2020.537290. eCollection 2020. Front Microbiol. 2020. PMID: 33224107 Free PMC article.
References
- Labischinski H. Med Microbiol Immunol. 1992;181:241–265. - PubMed
- Schneewind O, Fowler A, Faull K F. Science. 1995;268:103–106. - PubMed
- Mempel M, Schmidt T, Weidinger S, Schnopp C, Foster T, Ring J, Abeck D. J Invest Dermatol. 1998;111:452–456. - PubMed
- Foster T J, Mcdevitt D. FEMS Microbiol Lett. 1994;118:199–205. - PubMed
- Song M D, Wachi M, Doi M, Ishino F, Matsuhashi M. FEBS Lett. 1987;221:167–171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases