T helper cell type 2 cytokine-mediated comitogenic responses and CCR3 expression during differentiation of human mast cells in vitro - PubMed (original) (raw)
T helper cell type 2 cytokine-mediated comitogenic responses and CCR3 expression during differentiation of human mast cells in vitro
H Ochi et al. J Exp Med. 1999.
Abstract
Mast cells (MCs) arise in situ from circulating stem cell factor (SCF)-dependent committed progenitors (PrMCs) and accumulate at sites of allergic mucosal inflammation. We hypothesized that human (h)PrMCs and their mature counterparts might share overlapping patterns of chemokine and cytokine receptor utilization with eosinophils, basophils, and T helper type 2 (Th2) lymphocytes for their homing and allergy-associated hyperplasia. We have characterized committed hPrMCs and fully mature hMCs derived in vitro from cord blood for their functional responses to chemokine and cytokine agonists germane to allergic inflammation and for their maturation-related expression of the corresponding receptors. After 4 wk of culture in the presence of recombinant stem cell factor (SCF), interleukin (IL)-6, and IL-10, the cells were characterized as hPrMCs based upon their uniform surface expression of c-kit and CD13, low-level expression of FcinRIalpha, absence of CD14 and CD16 expression, and immunoreactivity for MC chymase in >80%, and about half were immunoreactive for tryptase and metachromatic with toluidine blue. By week 9, the cells had matured into hMCs, identified by higher levels of c-kit, continued expression of CD13 and low-level FcinRIalpha, uniform toluidine blue metachromasia, and uniform immunoreactivity for both tryptase and chymase. The 4-wk-old hPrMCs expressed four chemokine receptors (CXCR2, CCR3, CXCR4, and CCR5). Each receptor mediated transient rapid calcium fluxes in response to its respective ligand. Both recombinant human eotaxin and stromal cell-derived factor 1alpha elicited chemotaxis of hPrMCs. Only CCR3 was retained on the mature 9-wk-old hMCs from among these chemokine receptors, and hMCs responded to eotaxin with a sustained calcium flux but without chemotaxis. The Th2 cytokines IL-3, IL-5, IL-6, IL-9, and granulocyte/macrophage colony-stimulating factor each augmented the SCF-dependent proliferation of hPrMCs and hMCs. In contrast, the prototypical Th1 cytokine, interferon gamma, suppressed SCF-driven proliferation of both hPrMCs and hMCs. Thus, throughout their development in vitro, hMCs obey SCF-dependent, cytokine-driven mitogenic responses that reflect a Th2-type polarization characteristic of allergy and asthma. Furthermore, committed hPrMCs have a unique profile of chemokine receptor expression from among reported hematopoietic cells, including CCR3, which is shared with the other cells central to allergic inflammation (eosinophils, basophils, and Th2 lymphocytes).
Figures
Figure 1
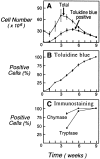
(A) Total cell numbers (•) and numbers of cells staining metachromatically with toluidine blue (▪) arising over 9 wk from cultures of 3 × 107 cord blood mononuclear cells in the presence of SCF/IL-6/IL-10. (B) Progressive increase in the percentage of cells with toluidine blue metachromasia. (C) Percentages of cells with tryptase (▪) and chymase immunoreactivity (•) at weeks 4, 6, and 9 of culture. Results are the means ± SEM of three experiments.
Figure 2
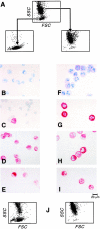
Cytofluorographic characteristics of 4- (top row) and 9-wk-old (second row) cells, each representing uniform populations by light scatter (left). Representative histograms are shown for c-kit, CD13, Fc∈RIα, IL-3Rα, and β3 integrin. At week 6, two cell subpopulations are indicated by distinct groups that differ in terms of the height of SSC. Separate gating and analysis revealed that the population of lesser SSC (low granularity; third row) resembled the 4-wk-old cells in its surface phenotype, whereas the higher SSC population (high granularity; fourth row) resembled the 9-wk-old cells. Expression of each surface epitope is represented by the bold tracings, and the isotype-matched negative controls are represented by the lighter tracings. Results are representative of independent experiments performed with cells from three different donors.
Figure 3
FACSorting of 6-wk-old cells into low and high granularity populations by their light scatter profiles (A). Toluidine blue metachromasia (B, F), tryptase immunoreactivity (C, G), chymase immunoreactivity (D, H), and chloroacetate esterase activity (E, I) are depicted for the low granularity (left) and high granularity (right) populations. Dot plot density light scatter profile of cells derived after 2 wk of culture from the original sorted 6-wk-old populations (J) of low granularity (left) and high granularity (right). The results are representative of two experiments, and the fields depict the profile presented quantitatively in the text.
Figure 4
Incorporation of [3H]thymidine by 4-wk-old hPrMCs (A) and 9-wk-old hMCs (B) that were cultured for six subsequent days in the presence of the indicated concentrations of cytokines (ng/ml). Results for individual cytokines (left) are expressed as the means ± SEM of triplicates and are representative of experiments performed with the cells of three different donors. The results for comitogenic responses (center) are expressed as the percentage increases above the responses to SCF alone and are the means ± SEM for three independent experiments. IFN-γ–mediated suppression of SCF-driven proliferation of hPrMCs (A, right; means ± half range, n = 2) and hMCs (B, right; means ± SEM, n = 3) is expressed as the percentage of thymidine incorporation occurring in response to 100 ng/ml of SCF alone.
Figure 5
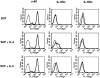
Reinduction of IL-3Rα on fully mature hMCs at week 9 of culture. hMCs were transferred to fresh medium containing SCF (100 ng/ml) alone (top row), SCF plus IL-3 (5 ng/ml; center row), or SCF plus IL-5 (5 ng/ml; bottom row). Cytofluorographic analysis was performed 1 wk later for the expression of c-kit (left column), IL-3Rα (center column), and IL-5Rα (right column). The results are representative of the two experiments performed.
Figure 6
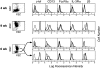
(A) Cytofluorographic expression of CXCR2, CCR3, CXCR4, CCR5, and CD4 by hPrMCs at week 4 (top row) and by hMCs at week 9 (bottom row). Expression of each surface epitope is represented by the bold tracings, and isotype-matched negative controls are represented by the lighter tracings. Results are representative of three experiments with different donor cells (n = 2 for CXCR2). (B) CCR3 (left) and CXCR4 (right) mRNA expression determined by RT-PCR analysis at 3, 4, 6, and 9 wk of culture compared with the internal standard, G3PDH.
Figure 6
(A) Cytofluorographic expression of CXCR2, CCR3, CXCR4, CCR5, and CD4 by hPrMCs at week 4 (top row) and by hMCs at week 9 (bottom row). Expression of each surface epitope is represented by the bold tracings, and isotype-matched negative controls are represented by the lighter tracings. Results are representative of three experiments with different donor cells (n = 2 for CXCR2). (B) CCR3 (left) and CXCR4 (right) mRNA expression determined by RT-PCR analysis at 3, 4, 6, and 9 wk of culture compared with the internal standard, G3PDH.
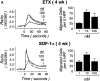
Figure 7
Functionality of CXCR4 and CCR3. Both ETX (A, top) and SDF-1α (A, bottom) caused transient dose-dependent calcium flux of Fura-loaded hPrMCs (left panels) and promoted their directed migration (right panels). At week 9, ETX elicited marked, sustained calcium flux (B) without chemotaxis (not shown). The calcium flux assays depicted are representative of three independent analyses at both weeks 4 and 9. The chemotaxis assays were analyzed by counting the numbers of migrating cells per five high power fields (HPF) and are the means ± SEM for three experiments.
Figure 7
Functionality of CXCR4 and CCR3. Both ETX (A, top) and SDF-1α (A, bottom) caused transient dose-dependent calcium flux of Fura-loaded hPrMCs (left panels) and promoted their directed migration (right panels). At week 9, ETX elicited marked, sustained calcium flux (B) without chemotaxis (not shown). The calcium flux assays depicted are representative of three independent analyses at both weeks 4 and 9. The chemotaxis assays were analyzed by counting the numbers of migrating cells per five high power fields (HPF) and are the means ± SEM for three experiments.
Similar articles
- The CCR3 receptor is involved in eosinophil differentiation and is up-regulated by Th2 cytokines in CD34+ progenitor cells.
Lamkhioued B, Abdelilah SG, Hamid Q, Mansour N, Delespesse G, Renzi PM. Lamkhioued B, et al. J Immunol. 2003 Jan 1;170(1):537-47. doi: 10.4049/jimmunol.170.1.537. J Immunol. 2003. PMID: 12496441 - The role of human mast cell-derived cytokines in eosinophil biology.
Shakoory B, Fitzgerald SM, Lee SA, Chi DS, Krishnaswamy G. Shakoory B, et al. J Interferon Cytokine Res. 2004 May;24(5):271-81. doi: 10.1089/107999004323065057. J Interferon Cytokine Res. 2004. PMID: 15153310 Review. - Role of human FcepsilonRI+ cells in HIV-1 infection.
Marone G, Florio G, Petraroli A, Triggiani M, de Paulis A. Marone G, et al. Immunol Rev. 2001 Feb;179:128-38. doi: 10.1034/j.1600-065x.2001.790113.x. Immunol Rev. 2001. PMID: 11292016 Review.
Cited by
- Suppression of CXCR4 expression in mast cells upon IgE-mediated antigen stimulation.
Matsuura J, Sakanaka M, Sato N, Ichikawa A, Tanaka S. Matsuura J, et al. Inflamm Res. 2010 Feb;59(2):123-7. doi: 10.1007/s00011-009-0078-7. Epub 2009 Aug 21. Inflamm Res. 2010. PMID: 19696965 - Effects of maternal exposure to di-(2-ethylhexyl) phthalate during fetal and/or neonatal periods on atopic dermatitis in male offspring.
Yanagisawa R, Takano H, Inoue K, Koike E, Sadakane K, Ichinose T. Yanagisawa R, et al. Environ Health Perspect. 2008 Sep;116(9):1136-41. doi: 10.1289/ehp.11191. Environ Health Perspect. 2008. PMID: 18795153 Free PMC article. - PGD2 deficiency exacerbates food antigen-induced mast cell hyperplasia.
Nakamura T, Maeda S, Horiguchi K, Maehara T, Aritake K, Choi BI, Iwakura Y, Urade Y, Murata T. Nakamura T, et al. Nat Commun. 2015 Jul 10;6:7514. doi: 10.1038/ncomms8514. Nat Commun. 2015. PMID: 26159556 - Leukotriene B4, an activation product of mast cells, is a chemoattractant for their progenitors.
Weller CL, Collington SJ, Brown JK, Miller HR, Al-Kashi A, Clark P, Jose PJ, Hartnell A, Williams TJ. Weller CL, et al. J Exp Med. 2005 Jun 20;201(12):1961-71. doi: 10.1084/jem.20042407. Epub 2005 Jun 13. J Exp Med. 2005. PMID: 15955837 Free PMC article. - Non-invasive optical imaging of eosinophilia during the course of an experimental allergic airways disease model and in response to therapy.
Markus MA, Dullin C, Mitkovski M, Prieschl-Grassauer E, Epstein MM, Alves F. Markus MA, et al. PLoS One. 2014 Feb 25;9(2):e90017. doi: 10.1371/journal.pone.0090017. eCollection 2014. PLoS One. 2014. PMID: 24587190 Free PMC article.
References
- Donaldson L.E., Schmitt E., Huntley J.F., Newlands G.F., Grencis R.K. A critical role for stem cell factor and c-kit in host protective immunity to an intestinal hel–minth. Int. Immunol. 1996;8:559–567. - PubMed
- Echtenacher B., Mannel D.N., Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–79. - PubMed
- O'Sullivan S., Dahlen B., Dahlen S.E., Kumlin M. Increased urinary excretion of the prostaglandin D2 metabolite 9α, 11 β-prostaglandin F2 after aspirin challenge supports mast cell activation in aspirin-induced airway obstruction. J. Allergy Clin. Immunol. 1996;98:421–432. - PubMed
- Castells M., Schwartz L.B. Tryptase levels in nasal lavage fluid as an indicator of the immediate allergic response. J. Allergy Clin. Immunol. 1988;82:348–355. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL036110/HL/NHLBI NIH HHS/United States
- U19 AI031599/AI/NIAID NIH HHS/United States
- AI-01304-02/AI/NIAID NIH HHS/United States
- AI-22532/AI/NIAID NIH HHS/United States
- AI-31599-06/AI/NIAID NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- P01 AI031599/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous