Differential susceptibility to hepatic inflammation and proliferation in AXB recombinant inbred mice chronically infected with Helicobacter hepaticus - PubMed (original) (raw)
Differential susceptibility to hepatic inflammation and proliferation in AXB recombinant inbred mice chronically infected with Helicobacter hepaticus
M Ihrig et al. Am J Pathol. 1999 Aug.
Abstract
Helicobacter hepaticus is a naturally occurring pathogen of mice and has been used to develop models of chronic hepatitis, liver cancer, and, more recently, inflammatory bowel disease, in selected mouse strains. A/JCr mice are particularly susceptible to H. hepaticus-induced hepatitis and subsequent development of liver neoplasms, whereas C57BL/6 mice are resistant. In this study, we inoculated nine AXB recombinant inbred (RI) mouse strains, derived from A/J and C57BL/6 mice, with H. hepaticus to determine the genetic basis of resistance to Helicobacter-induced liver disease. Mice were surveyed 14 months after inoculation by culture and PCR for H. hepaticus colonization of the liver and cecum, and microscopic morphometric evaluations of the liver were performed to quantify and correlate the severity of inflammation, apoptosis, and proliferation. Analysis of variance of hepatic inflammation demonstrated significant variation among the RI strains (P < 0.0001), and the strain distribution pattern suggested a multigenic basis of disease resistance. Quantitative trait analysis using linear regression suggested possible linkage to loci on mouse chromosome 19. Hepatocellular and biliary epithelial apoptosis and proliferation indices, including proliferation of oval cells, were markedly increased and correlated with severity of inflammation. Prevalence of hepatic neoplasia was also increased in susceptible RI strains. These findings demonstrate a genetic basis for susceptibility to Helicobacter-induced disease and provide insight into its pathogenesis.
Figures
Figure 1.
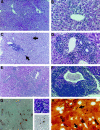
Representative photomicrographs of AXB RI strains demonstrating differential severity, distribution, and morphology of chronic inflammation 14 months after inoculation with H. hepaticus. A and B: Characteristic lesions of mild hepatitis with involvement of portal areas in relatively resistant RI strains 4 and 5, respectively. H&E, ×57 and ×114. C and D: Progressive chronic inflammation centered around veins and bile ductules in RI strains 2 and 10, respectively. Inflammation is chiefly lymphocytic, including numerous plasma cells but also contains scattered neutrophils and eosinophils and involves areas of parenchyma (arrows). H&E, ×57 and ×114. E and F: Severe lesions from RI strains 8 and 1, respectively. Inflammation has spread through the liver and is often associated with biliary epithelial hyperplasia and dysplasia. H&E, ×57 and ×95, respectively. G, Left: A RI strain 1 animal with severe hepatitis and high PCNA proliferation index also had pronounced oval cell proliferation, shown by immunostaining for A6 antibody. AEC, hematoxylin counterstain, ×95. G, Right: Hepatic parenchymal inflammation centered around apoptotic cells from AXB RI strain 8. H&E, ×370 (top), and TUNEL immunohistochemistry with hematoxylin counterstain, ×370 (bottom). H: Warthin-Starry stained liver section from RI strain 8, showing intercellular H. hepaticus organisms (arrows).
Figure 2.
A: Photomicrograph of AXB RI strain 1 male with hepatocellular adenoma that has compressed adjacent liver parenchyma (open arrows); variable degrees of cytoplasmic vacuolization and residual multifocal inflammation (solid arrows) are present within the neoplasm. H&E, ×31. The inset shows an area of lymphocytic inflammation surrounding poorly organized biliary epithelium within neoplasm (lower arrow). H&E, ×165. B: Hepatocellular carcinoma with irregular cords and clusters of large, pleomorphic hepatocytes and foci of lymphocytic inflammation from AXB RI strain 6 male. H&E, ×100. C: AXB RI strain 12 female with B-cell lymphosarcoma that has diffusely infiltrated an area of large intestine from the muscularis mucosa (mm) to the superficial epithelium (left). H&E, ×78. The same animal had lymphosarcoma within the liver (right). A remaining bile ductule is present with the neoplasm (white arrow). H&E, ×160. D: AXB RI strain 1 female with severe hepatitis and development of poorly differentiated hemangiosarcoma lining sinusoids and vascular channels. H&E, ×160.
Figure 3.
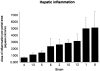
Geometric means and standard errors for the morphometric scores of hepatic inflammation in the nine AXB recombinant inbred mouse strains. The surface area affected by inflammation was determined for each mouse by examination of three liver sections prepared from samples collected in a standardized manner. The number of 50 μm boxes in an ocular grid filled with inflammatory cells was counted, and the area of those counted boxes was calculated. The number of portal areas contained within the three liver sections was used as an estimate of total surface area of the three liver sections. The area calculated from the boxes filled with inflammatory cells (surface area of inflammation) was divided by the number of portal areas counted (total surface area estimate) to give the area affected by inflammation per portal area in square microns.
Figure 4.
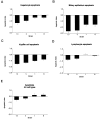
The apoptotic index for the four AXB recombinant inbred strains assessed for apoptosis. The TUNEL procedure was performed on liver sections from four RI strains with the most extreme inflammation phenotypes. The positively stained cells in liver sections were counted and categorized, the total surface area of the liver sections was determined with image analysis software, and the number of positive cells was divided by the total surface area. The data were then log transformed to obtain the apoptotic index. The graphs represent the mean apoptotic indices for hepatocytes (A), bile duct epithelial cells (B), Kupffer cells (C), lymphocytes (D), and all cell types combined (E).
Figure 5.
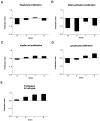
The proliferation index for the four AXB recombinant inbred strains assessed for proliferation. Liver sections from four RI strains with the most extreme inflammation phenotypes were immunostained with PCNA antibody. The positively stained cells in liver sections were counted and categorized, the total surface area of the liver sections was determined with image analysis software, and the number of positive cells was divided by the total surface area. The data were then log transformed to obtain the proliferation index. The graphs represent the mean proliferation indices for hepatocytes (A), bile duct epithelial cells (B), Kupffer cells (C), lymphocytes (D), and all cell types combined (E).
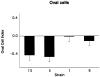
Figure 6.
The oval cell index for the four AXB recombinant inbred strains assessed for oval cell hyperplasia. Liver sections from four RI strains with the most extreme inflammation phenotypes were immunostained with A6 antibody, which is specific for oval cells. The positively stained cells in liver sections were counted, the surface area of the liver sections was determined with image analysis software, and the number of positive cells was divided by the total surface area. The data were then log transformed to obtain the oval cell index. The graphs represent the mean oval cell indices for the four RI strains.
Similar articles
- Chronic proliferative hepatitis in A/JCr mice associated with persistent Helicobacter hepaticus infection: a model of helicobacter-induced carcinogenesis.
Fox JG, Li X, Yan L, Cahill RJ, Hurley R, Lewis R, Murphy JC. Fox JG, et al. Infect Immun. 1996 May;64(5):1548-58. doi: 10.1128/iai.64.5.1548-1558.1996. Infect Immun. 1996. PMID: 8613359 Free PMC article. - Genetic susceptibility to chronic hepatitis is inherited codominantly in Helicobacter hepaticus-infected AB6F1 and B6AF1 hybrid male mice, and progression to hepatocellular carcinoma is linked to hepatic expression of lipogenic genes and immune function-associated networks.
García A, Ihrig MM, Fry RC, Feng Y, Xu S, Boutin SR, Rogers AB, Muthupalani S, Samson LD, Fox JG. García A, et al. Infect Immun. 2008 May;76(5):1866-76. doi: 10.1128/IAI.01044-07. Epub 2008 Feb 19. Infect Immun. 2008. PMID: 18285497 Free PMC article. - Liver tumorigenesis by Helicobacter hepaticus: considerations of mechanism.
Canella KA, Diwan BA, Gorelick PL, Donovan PJ, Sipowicz MA, Kasprzak KS, Weghorst CM, Snyderwine EG, Davis CD, Keefer LK, Kyrtopoulos SA, Hecht SS, Wang M, Anderson LM, Rice JM. Canella KA, et al. In Vivo. 1996 May-Jun;10(3):285-92. In Vivo. 1996. PMID: 8797029 Review. - Hepatocarcinogenesis: a polygenic model of inherited predisposition to cancer.
Dragani TA, Canzian F, Manenti G, Pierotti MA. Dragani TA, et al. Tumori. 1996 Jan-Feb;82(1):1-5. doi: 10.1177/030089169608200101. Tumori. 1996. PMID: 8623496 Review.
Cited by
- No evidence of Helicobacter pylori sequences in pancreatic juices of patients affected by chronic pancreatitis.
Di Campli C, Nocente R, Costamagna G, Gentiloni N, Burioni R, Wu J, Armuzzi A, Zern MA, Gasbarrini G, Gasbarrini A. Di Campli C, et al. Int J Pancreatol. 2000 Dec;28(3):181-5. doi: 10.1385/IJGC:28:3:181. Int J Pancreatol. 2000. PMID: 11373055 - CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice.
Erdman SE, Poutahidis T, Tomczak M, Rogers AB, Cormier K, Plank B, Horwitz BH, Fox JG. Erdman SE, et al. Am J Pathol. 2003 Feb;162(2):691-702. doi: 10.1016/S0002-9440(10)63863-1. Am J Pathol. 2003. PMID: 12547727 Free PMC article. - Helicobacter hepaticus urease is not required for intestinal colonization but promotes hepatic inflammation in male A/JCr mice.
Ge Z, Lee A, Whary MT, Rogers AB, Maurer KJ, Taylor NS, Schauer DB, Fox JG. Ge Z, et al. Microb Pathog. 2008 Jul;45(1):18-24. doi: 10.1016/j.micpath.2008.02.003. Epub 2008 Mar 27. Microb Pathog. 2008. PMID: 18486436 Free PMC article. - Quantitative trait loci in a bacterially induced model of inflammatory bowel disease.
Hillhouse AE, Myles MH, Taylor JF, Bryda EC, Franklin CL. Hillhouse AE, et al. Mamm Genome. 2011 Oct;22(9-10):544-55. doi: 10.1007/s00335-011-9343-5. Epub 2011 Jun 30. Mamm Genome. 2011. PMID: 21717222 Free PMC article. - Simple epithelium keratins 8 and 18 provide resistance to Fas-mediated apoptosis. The protection occurs through a receptor-targeting modulation.
Gilbert S, Loranger A, Daigle N, Marceau N. Gilbert S, et al. J Cell Biol. 2001 Aug 20;154(4):763-73. doi: 10.1083/jcb.200102130. J Cell Biol. 2001. PMID: 11514590 Free PMC article.
References
- Ward JM, Fox JG, Anver MR, Haines DC, George CV, Collins MJ, Jr, Gorelick PL, Nagashima K, Gonda MA, Gilden RV, Tully JG, Russell RJ, Benveniste RE, Paster BJ, Dewhirst FE, Donovan JC, Anderson LM, Rice JM: Chronic active hepatitis and associated liver tumors in mice caused by a persistent bacterial infection with a novel Helicobacter species. J Natl Cancer Inst 1994, 86:1222-1227 - PubMed
- Canella KA, Diwan BA, Gorelick PL, Donovan PJ, Sipowicz MA, Kasprzak KS, Weghorst CM, Snyderwine EG, Davis CD, Keefer LK, Kyrtopoulos SA, Hecht SS, Wang M, Anderson LM, Rice JM: Liver tumorigenesis by Helicobacter hepaticus: considerations of mechanism. In Vivo 1996, 10:285-292 - PubMed
- Steininger H, Faller G, Dewald E, Brabletz T, Jung A, Kirchner T: Apoptosis in chronic gastritis and its correlation with antigastric autoantibodies. Virchows Arch 1998, 433:13-18 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases