Long-term enhancement of central synaptic transmission by chronic brain-derived neurotrophic factor treatment - PubMed (original) (raw)
Long-term enhancement of central synaptic transmission by chronic brain-derived neurotrophic factor treatment
N T Sherwood et al. J Neurosci. 1999.
Abstract
Acute effects of neurotrophins on synaptic plasticity have recently received much attention, but the roles of these factors in regulating long-lasting changes in synaptic function remain unclear. To address this issue we studied the long-term (days to weeks) and short-term (minutes to hours) effects of brain-derived neurotrophic factor (BDNF) on excitatory synaptic transmission in autaptic cultures of hippocampal CA1 neurons. We found that BDNF induced long-term enhancement of the strength of non-NMDA receptor-mediated glutamatergic transmission. This upregulation of EPSC amplitude occurred via an increase in the size of unitary synaptic currents, with no significant contribution from other aspects of neuronal electrical and synaptic function including cell size, voltage-gated sodium and potassium current levels, the number and size of synaptic contacts, and the frequency of spontaneous neurotransmitter release. Chronic BDNF treatment also decreased the degree of synaptic depression measured in response to paired stimuli. Thus, BDNF induced long-term synaptic enhancement of both basal and use-dependent synaptic transmission via specific changes to the synapse rather than through generalized potentiation of neuronal growth and differentiation. Finally, we showed that the long-term effects of BDNF are functionally and mechanistically distinct from its acute effects on synaptic transmission, suggesting that, in vivo, BDNF activation of Trk receptors can have different functional effects depending on the time course of its action.
Figures
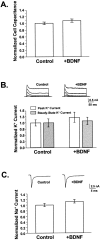
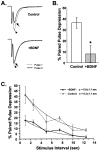
Fig. 1.
Chronic BDNF treatment increased the amplitude of evoked non-NMDA-type postsynaptic currents. A,Top, EPSCs were elicited by depolarizing CA1 autaptic neurons from a prepulse potential of −120 mV to +10 mV for 1.6 msec and returning to −70 mV, causing a rapid inward sodium spike (arrow) and subsequent EPSC (arrowhead). Recorded EPSCs could be completely blocked by NBQX, leaving only a residual Na+ current (center). The NBQX-sensitive component of such traces (bottom; difference of top and center traces) defined non-NMDA-type glutamate receptor-mediated synaptic currents that reversed at or near 0 mV. B, C, Chronic BDNF treatment (100 ng/ml; n = 61) increased average EPSC peak amplitude by 1.7 ± 0.1-fold (mean ± SE) compared with those recorded from controls (i.e., untreated or 10 μg/ml TrkB–IgG-treated neurons;n = 109; p < 8.8 × 10−8 by ANOVA; statistically significant difference denoted by the asterisk). Representative EPSCs are shown in B. Mean EPSC amplitudes (C) ranged from ∼1 to 4 nA and were normalized to controls within each experiment to compare results from different platings.
Fig. 2.
BDNF did not appreciably affect neuronal size or membrane excitability. A, BDNF did not affect cell size. Cell capacitance was used to measure total membrane area, which did not differ between neurons grown in the presence or absence of added BDNF (p > 0.15). Average capacitances ranged from 40 to 60 pF (Control, n = 85;+BDNF, n = 41). B, BDNF did not affect potassium currents. Voltage-gated potassium currents were elicited by depolarizing neurons from a 20 msec prepulse of −120 mV to between −40 and +40 mV in 20 mV increments for 200 msec; representative traces are shown for control and BDNF-treated cells. Currents at +40 mV were measured at peak and at steady state to reflect the contributions of transient and sustained potassium current components. No differences were observed between untreated controls and neurons grown in BDNF for either of these measurements. Average potassium currents were ∼2 nA. Control,n = 10; +BDNF, _n_= 9. p > 0.35 for both peak and steady-state current comparisons. C, BDNF did not alter sodium currents. Whole-cell sodium currents were recorded by depolarizing neurons to −10 mV from a brief hyperpolarizing pulse of −120 mV; representative traces are shown. Sodium currents activated and inactivated within 5 msec after the start of the depolarizing pulse. Average peak sodium currents were equivalent in BDNF-treated neurons and controls (n = 41 and 60, respectively;p > 0.09) and ranged from 4 to 8 nA.
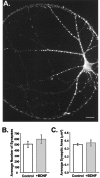
Fig. 3.
Synaptogenesis was not affected by chronic BDNF treatment. A, Fluorescence image of an autaptic neuron labeled with an antibody directed against synapsin Ia,b and visualized using a Texas Red-conjugated secondary antibody. Each bright puncta represents a presynaptic specialization. Scale bar, 20 μm.B, The number of synapses per neuron did not differ between treatment groups. Average numbers of synapses per cell were (mean ± SE) 506 ± 58 in controls and 594 ± 74 in BDNF-treated neurons (n = 13 and 7, respectively;p > 0.37). C, The average size of synapses was unaffected by BDNF. Mean synapse size, with each synapse defined as the pixel area of an isolated puncta, was 0.35 ± 0.01 and 0.37 ± 0.04 μm2 per synapse for control (n = 13) and +BDNF (n = 7) cells, respectively; p > 0.56.
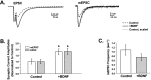
Fig. 4.
BDNF increased EPSC and mEPSC amplitudes proportionally but did not affect mEPSC frequency. A, EPSC (left) and mEPSC (right) traces were averaged for control (dashed traces) and +BDNF–treated (solid traces) cells and superimposed. BDNF increased EPSCs and mEPSCs to similar extents. Scaling the averaged control traces to their respective +BDNF traces (dotted lines) showed that the kinetics of activation and decay were indistinguishable between treatment groups for both evoked and spontaneous responses.B, EPSC amplitudes (shaded bars) were 1.8 ± 0.03-fold greater in BDNF-treated cells compared with controls (p < 0.02; _+BDNF_,_n_ = 13; _Control_,_n_ = 16). BDNF increased the amplitudes of mEPSCs to the same extent (1.8 ± 0.02-fold; _p_ < 0.03;_+BDNF_, _n_ = 10;_Control_, _n_ = 12). Mean EPSC amplitudes were 3.6 and 2.0 nA for +BDNF and control cells, respectively, whereas mEPSC amplitudes averaged 34.2 and 18.6 pA. Values normalized to control cells are shown; _asterisks_denote statistical significance. _C_, In contrast, BDNF did not affect the average frequency of mEPSCs (_+BDNF_,_n_ = 10; _Control_,_n_ = 12; _p_ > 0.25). mEPSCs were measured from 51.2 sec of continuous recording while cells were held at −70 mV.
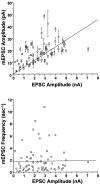
Fig. 5.
mEPSC amplitude, but not frequency, covaried with EPSC amplitude in populations of BDNF-treated and control neurons.Top, Amplitudes of spontaneous and evoked postsynaptic currents covaried with a correlation coefficient of_r_ = 0.57 (p < 1 × 10−4); best linear least-squares fit is shown (_solid line_). Each point represents recordings from a single neuron; mEPSC amplitudes are the mean ± SE of all events occurring within the 51.2 sec recording period. Two sources of variation in these data points were evident. (1) Limitations in the signal-to-noise of some recordings reduced the detectability of mEPSCs smaller than ∼3 pA, leading to an overestimation of average mEPSC amplitudes in these neurons. Hence, there is more scatter above the linear fit than below it. (2) Although BDNF never altered the quantal content of neurons within a given cell culture plating, there was some variation in overall quantal content from plating to plating (122–225). Thus, the slope of the linear fit here reflects the weighted average of the quantal contents of all neurons in all platings (178). It is noteworthy that the strong correlation between mEPSCs and EPSC amplitudes remained clear despite these significant sources of experimental variation. _Bottom_, In contrast, a plot of mEPSC frequency versus EPSC amplitude for individual cells revealed no correlation: _r_ = 0.01, _p_ > 0.9. n = 36 and 30 for control (○) and BDNF-treated (■) cells, respectively.
Fig. 6.
Chronic BDNF treatment attenuated paired-pulse depression. A, Sample traces of EPSCs recorded using a paired-pulse depression (PPD) paradigm for cells grown in the presence or absence of exogenous BDNF. The dotted traces(arrowhead) show the first evoked postsynaptic current.Solid traces show the second EPSC (arrow) evoked 1 sec later. In the BDNF-treated neuron the two traces are nearly overlapping, indicating that little PPD occurred. In contrast, in the control neuron the second EPSC was substantially depressed relative to the first. B, On average, control neurons showed 36 ± 5% PPD at an interpulse interval of 1 sec (n = 13). In contrast, BDNF-treated cells showed only 8 ± 8% PPD (n = 12;p < 0.005; statistical significance denoted by_asterisk_). C, The rate of recovery from PPD was not affected by BDNF. PPD was measured for a range of interpulse intervals ranging from 1 to 12.5 sec. The plot of PPD versus interpulse interval shows that control neurons were more depressed than BDNF-treated neurons at all intervals. The time courses of recovery from PPD, however, were fitted well by single exponential functions with similar time constants (Control, 6.3 ± 1.1 sec, n = 12; +BDNF, 5.8 ± 1.1 sec, n = 8; fits constrained to be asymptotic to zero).
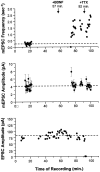
Fig. 7.
Acute application of BDNF increased only the frequency of mEPSCs. In this example, synaptic currents from a single autaptic neuron were first recorded in the absence of BDNF to establish baseline synaptic transmission properties (mEPSC frequency, mEPSC amplitude, and EPSC amplitude). Each mEPSC point represents 25.6 sec of recording. BDNF was then added to the bath (100 ng/ml at_t_ = 57 min), and these synaptic properties were monitored for 35 min. Finally, TTX was added at t = 92 min to confirm that the mEPSCs measured were true action potential-independent minis. Top, The frequency of mEPSCs increased 5.2-fold (p < 2 × 10−9) in response to BDNF addition. This effect was seen within minutes and was not affected by the subsequent addition of TTX (_p_ > 0.1, comparing average post-BDNF frequencies measured before and after TTX).Center, No differences were observed in average mEPSC amplitudes (±SE) throughout the recording; p > 0.9 for mEPSC amplitudes measured before and after BDNF addition. Blank periods in this and the graph above are due to EPSCs being recorded during these intervals. Bottom, Similarly, EPSCs were unaffected by the addition of BDNF (p > 0.8, comparing pre-BDNF and post-BDNF measurements). Note that the last two EPSC amplitudes are zero, confirming that sodium channels were blocked by TTX, eliminating all action potentials and EPSCs.
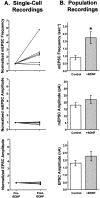
Fig. 8.
Acute application of BDNF increased mEPSC frequency but not mEPSC or EPSC amplitudes. A, Summary of single-cell neuron recordings. Each symbol represents an individual neuron recorded continuously, first in control salines and then for up to 80 min after the addition of BDNF. Values are normalized to the control (pre-BDNF) measurement for each neuron.Top, mEPSC frequency increased significantly by 10 min after the addition of BDNF, by an average of 2.5-fold (n = 6; p < 0.006). This increase was insensitive to TTX, indicating that BDNF increased the frequency of true minis (Fig. 7 and data not shown)._Center_, mEPSC amplitudes were unchanged by BDNF addition (_n_ = 6). _Bottom_, EPSC amplitudes were also unaffected by short-term BDNF treatment (_n_ = 7). _B_, Summary of data taken from populations of neurons untreated or treated with BDNF for <2.5 hr. _Top_, As in the single cell experiments, BDNF increased mEPSC frequency by 2.5-fold (_Control_, 0.88 ± 0.14 sec−1,_n_ = 24; _+BDNF_, 2.23 ± 0.39 sec−1, _n_ = 18; _p_ < 0.001; significance denoted by_asterisk_). _Center_, mEPSC amplitudes were indistinguishable between control and BDNF-treated cells (_Control_, _n_ = 26;_+BDNF_, _n_ = 19; _p_> 0.1). Bottom, Similarly, evoked postsynaptic currents did not differ significantly in amplitude between control and BDNF-treated neurons (Control, n = 27; +BDNF, n = 22;p > 0.17).
Similar articles
- Enhancement of neurotransmitter release induced by brain-derived neurotrophic factor in cultured hippocampal neurons.
Li YX, Zhang Y, Lester HA, Schuman EM, Davidson N. Li YX, et al. J Neurosci. 1998 Dec 15;18(24):10231-40. doi: 10.1523/JNEUROSCI.18-24-10231.1998. J Neurosci. 1998. PMID: 9852560 Free PMC article. - Presynaptic and postsynaptic NMDA receptors mediate distinct effects of brain-derived neurotrophic factor on synaptic transmission.
Madara JC, Levine ES. Madara JC, et al. J Neurophysiol. 2008 Dec;100(6):3175-84. doi: 10.1152/jn.90880.2008. Epub 2008 Oct 15. J Neurophysiol. 2008. PMID: 18922945 Free PMC article. - Neurotrophin-dependent modulation of glutamatergic synaptic transmission in the mammalian CNS.
Lessmann V. Lessmann V. Gen Pharmacol. 1998 Nov;31(5):667-74. doi: 10.1016/s0306-3623(98)00190-6. Gen Pharmacol. 1998. PMID: 9809461 Review. - Long-term regulation of excitatory and inhibitory synaptic transmission in hippocampal cultures by brain-derived neurotrophic factor.
Bolton MM, Lo DC, Sherwood NT. Bolton MM, et al. Prog Brain Res. 2000;128:203-18. doi: 10.1016/s0079-6123(00)28018-7. Prog Brain Res. 2000. PMID: 11105680 Review. No abstract available.
Cited by
- Oligodendrocytes regulate presynaptic properties and neurotransmission through BDNF signaling in the mouse brainstem.
Jang M, Gould E, Xu J, Kim EJ, Kim JH. Jang M, et al. Elife. 2019 Apr 18;8:e42156. doi: 10.7554/eLife.42156. Elife. 2019. PMID: 30998186 Free PMC article. - Expression of cocaine- and amphetamine-regulated transcript in the rat forebrain during postnatal development.
Rodrigues BC, Cavalcante JC, Elias CF. Rodrigues BC, et al. Neuroscience. 2011 Nov 10;195:201-14. doi: 10.1016/j.neuroscience.2011.08.036. Epub 2011 Aug 30. Neuroscience. 2011. PMID: 21903152 Free PMC article. - The role of neurotrophic factors in novel, rapid psychiatric treatments.
Kim J, He MJ, Widmann AK, Lee FS. Kim J, et al. Neuropsychopharmacology. 2024 Jan;49(1):227-245. doi: 10.1038/s41386-023-01717-x. Epub 2023 Sep 6. Neuropsychopharmacology. 2024. PMID: 37673965 Free PMC article. Review. - Brain-derived neurotrophic factor (BDNF) Val(66)Met polymorphism differentially predicts hippocampal function in medication-free patients with schizophrenia.
Eisenberg DP, Ianni AM, Wei SM, Kohn PD, Kolachana B, Apud J, Weinberger DR, Berman KF. Eisenberg DP, et al. Mol Psychiatry. 2013 Jun;18(6):713-20. doi: 10.1038/mp.2012.187. Epub 2013 Jan 15. Mol Psychiatry. 2013. PMID: 23319002 Free PMC article. - Brain-derived neurotrophic factor enhances GABA release probability and nonuniform distribution of N- and P/Q-type channels on release sites of hippocampal inhibitory synapses.
Baldelli P, Hernandez-Guijo JM, Carabelli V, Carbone E. Baldelli P, et al. J Neurosci. 2005 Mar 30;25(13):3358-68. doi: 10.1523/JNEUROSCI.4227-04.2005. J Neurosci. 2005. PMID: 15800191 Free PMC article.
References
- Bekkers JM, Stevens CF. Presynaptic mechanism for long-term potentiation in the hippocampus. Nature. 1990;346:724–729. - PubMed
- Bekkers JM, Stevens CF. Quantal analysis of EPSCs recorded from small numbers of synapses in hippocampal cultures. J Neurophysiol. 1995;73:1145–1156. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous