Regulation of the iron regulatory proteins by reactive nitrogen and oxygen species - PubMed (original) (raw)
Review
Regulation of the iron regulatory proteins by reactive nitrogen and oxygen species
E S Hanson et al. Gene Expr. 1999.
Abstract
Iron regulatory proteins 1 and 2 (IRP1 and IRP2) are RNA binding proteins that posttranscriptionally regulate the expression of mRNAs coding for proteins involved in the maintenance of iron and energy homeostasis. The RNA binding activities of the IRPs are regulated by changes in cellular iron. Thus, the IRPs are considered iron sensors and the principle regulators of cellular iron homeostasis. The mechanisms governing iron regulation of the IRPs are well described. Recently, however, much attention has focused on the regulation of IRPs by reactive nitrogen and oxygen species (RNS, ROS). Here we focus on summarizing the iron-regulated RNA binding activities of the IRPs, as well as the recent findings of IRP regulation by RNS and ROS. The recent observations that changes in oxygen tension regulate both IRP1 and IRP2 RNA binding activities will be addressed in light of ROS regulation of the IRPs.
Figures
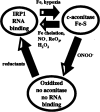
FIG. 1
Model for the regulation of IRP1 by iron, ROS, and RNS. IRP1 interconverts between a RNA binding form and a [4Fe-4S] c-aconitase form. Iron or hypoxia converts the apo-RNA binding form into the [4Fe-4S] aconitase form. NO•, H2O2, iron chelation, or reoxygenation (ReO2) results in the formation of the IRP1 RNA binding form. ONNO– results in the disassembly of the [4Fe-4S] cluster and oxidation of cysteines required for RNA binding. This results in the formation of an oxidized protein, lacking RNA binding and c-aconitase activities. Addition of reductants to oxidized IRP1 restores RNA binding activity.
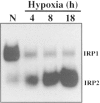
FIG. 2
Hypoxic regulation of IRP1 and IRP2 Hepa-1 cells. Mouse Hepa-1 clc4 were exposed to normoxia (N) or hypoxia (1% O2) for the indicated times. Bandshift analysis was performed by incubating cytosolic extracts (12 μg) with a 32P-labeled iron responsive element RNA probe. The RNA–protein complexes were resolved on a 5% nondenaturing polyacrylamide gel, and the gel was exposed to film. IRP1–RNA and IRP2–RNA complexes are indicated.
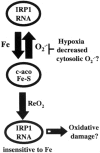
FIG. 3
Model depicting oxygen regulation of IRP1. During normoxia IRP1 interconverts between its RNA binding form and its [4Fe-4S] c-aconitase form. This interconversion is dependent on the relative levels of iron and O2 •–. Hypoxia decreases RNA binding activity (see Fig. 2) and increases c-aconitase activity. The model suggests that hypoxic regulation of IRP1 could be due to increased iron and/or decreased cytosolic O2 •–, either of which would lead to stabilization of the [4Fe-4S] cluster at the expense of RNA binding activity. Reoxygenation (ReO2) activates IRP1 to a constitutively active RNA binding form. The dysregulated form of IRP1 (shaded) is refractory to iron downregulation. By adversely affecting iron levels, it is possible that this form of IRP1 may contribute to ReO2-induced oxidative damage.
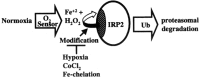
FIG. 4
Model for the oxygen regulation of IRP2. Normoxic degradation of IRP2 by the proteasome is dependent on the 73-amino acid degradation domain that contains three essential cysteines required to sense Fe2+ (hashed) (51). In this model, IRP2 stability is dependent on Fe2+ and H2O2, which are required for oxidative modification leading to ubiquitination (Ub) and proteasomal degradation (50). Hypoxia, iron chelation, and CoCl2 increase IRP2 protein levels by stabilization. Hypoxia may act through an unknown O2 sensor that lowers cytosolic H2O2 resulting in decreased IRP2 oxidation and degradation. CoCl2 mimics hypoxia by possibly altering the activity of an O2 sensor or by competing with iron at an iron binding site on the degradation domain.
Similar articles
- Differential regulation of IRP1 and IRP2 by nitric oxide in rat hepatoma cells.
Phillips JD, Kinikini DV, Yu Y, Guo B, Leibold EA. Phillips JD, et al. Blood. 1996 Apr 1;87(7):2983-92. Blood. 1996. PMID: 8639920 - Expression and biochemical characterization of iron regulatory proteins 1 and 2 in Saccharomyces cerevisiae.
Phillips JD, Guo B, Yu Y, Brown FM, Leibold EA. Phillips JD, et al. Biochemistry. 1996 Dec 10;35(49):15704-14. doi: 10.1021/bi960653l. Biochemistry. 1996. PMID: 8961933 - Iron regulatory proteins, iron responsive elements and iron homeostasis.
Eisenstein RS, Blemings KP. Eisenstein RS, et al. J Nutr. 1998 Dec;128(12):2295-8. doi: 10.1093/jn/128.12.2295. J Nutr. 1998. PMID: 9868172 Review. - Nitrogen monoxide-mediated control of ferritin synthesis: implications for macrophage iron homeostasis.
Kim S, Ponka P. Kim S, et al. Proc Natl Acad Sci U S A. 2002 Sep 17;99(19):12214-9. doi: 10.1073/pnas.192316099. Epub 2002 Sep 3. Proc Natl Acad Sci U S A. 2002. PMID: 12209009 Free PMC article. - Redox control of iron regulatory proteins.
Fillebeen C, Pantopoulos K. Fillebeen C, et al. Redox Rep. 2002;7(1):15-22. doi: 10.1179/135100002125000136. Redox Rep. 2002. PMID: 11981450 Review.
Cited by
- Aconitate hydratase of mammals under oxidative stress.
Matasova LV, Popova TN. Matasova LV, et al. Biochemistry (Mosc). 2008 Sep;73(9):957-64. doi: 10.1134/s0006297908090010. Biochemistry (Mosc). 2008. PMID: 18976211 Free PMC article. Review. - Early and late molecular events in neurodegeneration and neuroprotection in Parkinson's disease MPTP model as assessed by cDNA microarray; the role of iron.
Youdim MB, Grünblatt E, Levites Y, Maor G, Mandel S. Youdim MB, et al. Neurotox Res. 2002 Nov-Dec;4(7-8):679-689. doi: 10.1080/1029842021000045507. Neurotox Res. 2002. PMID: 12709306 - Dysregulation of the sensory and regulatory pathways controlling cellular iron metabolism in unilateral obstructive nephropathy.
Votava JA, Reese SR, Deck KM, Nizzi CP, Anderson SA, Djamali A, Eisenstein RS. Votava JA, et al. Am J Physiol Renal Physiol. 2022 Jan 1;322(1):F89-F103. doi: 10.1152/ajprenal.00537.2020. Epub 2021 Nov 29. Am J Physiol Renal Physiol. 2022. PMID: 34843656 Free PMC article. - Cellular Dynamics of Transition Metal Exchange on Proteins: A Challenge but a Bonanza for Coordination Chemistry.
Moulis JM. Moulis JM. Biomolecules. 2020 Nov 21;10(11):1584. doi: 10.3390/biom10111584. Biomolecules. 2020. PMID: 33233467 Free PMC article. Review. - Comparative effects of doxorubicin and a doxorubicin analog, 13-deoxy, 5-iminodoxorubicin (GPX-150), on human topoisomerase IIβ activity and cardiac function in a chronic rabbit model.
Frank NE, Cusack BJ, Talley TT, Walsh GM, Olson RD. Frank NE, et al. Invest New Drugs. 2016 Dec;34(6):693-700. doi: 10.1007/s10637-016-0388-x. Epub 2016 Aug 31. Invest New Drugs. 2016. PMID: 27581956
References
- Addess K. J.; Basilion J. P.; Klausner R. D.; Rou-ault T. A.; Pardi A. Structure and dynamics of the iron responsive element RNA: Implications for binding of the RNA by iron regulatory binding proteins. J. Mol. Biol. 274:72–83; 1997. - PubMed
- Aisen P.; Wessling-Resnick M.; Leibold E. A. Iron metabolism. Curr. Opin. Chem. Biol. 3:200–206; 1999. - PubMed
- Andrews N. C. ; Levy J. E. Iron is hot: An update on the pathophysiology of hemochromatosis. Blood 92: 1845–1851; 1998. - PubMed
- Beinert H.; Kennedy M. C. Aconitase, a two-faced protein: Enzyme and iron regulatory factor. FASEB J. 7:1442–1449; 1993. - PubMed
- Berlett B. S.; Stadtman E. R. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 272:20313–20316; 1997. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources