Independent regulation of flowering by phytochrome B and gibberellins in Arabidopsis - PubMed (original) (raw)
Independent regulation of flowering by phytochrome B and gibberellins in Arabidopsis
M A Blázquez et al. Plant Physiol. 1999 Aug.
Abstract
Phytochromes and gibberellins (GAs) coordinately regulate multiple aspects of Arabidopsis development. Phytochrome B (PHYB) promotes seed germination by increasing GA biosynthesis, but inhibits hypocotyl elongation by decreasing the responsiveness to GAs. Later in the life cycle of the plant, PHYB and GAs have opposite effects on flowering. PHYB delays flowering, while GAs promote flowering, particularly under noninductive photoperiods. To learn how PHYB and GAs interact in the control of flowering, we have analyzed the effect of a phyB mutation on flowering time and on the expression of the floral meristem-identity gene LFY (LEAFY). We show that the early flowering caused by phyB correlated with an increase in LFY expression, which complements our previous finding that GAs are required for activation of LFY under noninductive photoperiods (M.A. Blázquez, R. Green, O. Nilsson, M.R. Sussman, D. Weigel [1998] Plant Cell 10: 791-800). Since phyB did not change the GA responsiveness of the LFY promoter and suppressed the lack of flowering of severe GA-deficient mutants under short days, we propose that PHYB modulates flowering time at least partially through a GA-independent pathway. Interestingly, the effects of PHYB on flowering do not seem to be mediated by transcriptional up-regulation of genes such as CO (CONSTANS) and FT (Flowering locus T), which are known to mediate the effects of the photoperiod-dependent floral-induction pathway.
Figures
Figure 1
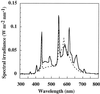
Light spectrum inside the growth chambers used in this work. The solid black line represents the spectrum provided by a 3:1 mixture of cool-white to fluorescent light, while the dotted line represents the spectrum provided by cool-white light alone.
Figure 2
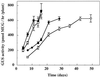
LFY::GUS expression during vegetative growth of _phyB_-5 mutants. Plants homozygous for the LFY::GUS transgene either in a PHYB+ (DW150-304; white symbols) or _phyB_-5 background (307B5; black symbols) were grown in long days (squares) or short days (circles) until flower buds were visible to the naked eye. Values are expressed as means ± 2
se
(n = 12). Time represents days after sowing. Error bars that are not visible are contained within the symbol. MUG, 4-Methylumbelliferyl-β-
d
-glucopyranoside.
Figure 3
Suppression of the _ga1_-3 flowering defect in short days by _phyB_-5. Representative plants were photographed 50 d after sowing. Arrowhead indicates flowers.
Figure 4
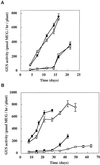
LFY::GUS expression during vegetative growth of _ga1_-3 and _ga1_-_3 phyB_-5 mutants. Plants homozygous for the LFY::GUS transgene were grown in long (A) or short (B) days until flower buds were visible to the naked eye, except in the case of the _ga1_-3 mutant without GA3 treatment under short days (□), which had not flowered at the end of the experiment. 304G1 (_ga1_-3, white symbols) and 304G1B5 (_ga1_-_3 phyB_-5, black symbols) were treated with GA3 (circles) or left untreated (squares). Values are expressed as means ± 2
se
(n = 12). Time represents days after sowing. Error bars that are not visible are contained within the symbol. MUG, 4-Methylumbelliferyl-β-
d
-glucopyranoside.
Figure 5
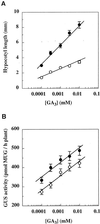
Responses of _ga1_-3 and _ga1_-_3 phyB_-5 seedlings to exogenous GA3. Seeds of the lines 304G1 (_LFY::GUS ga1_-3, ○) and 304G1B5 (_LFY::GUS ga1_-_3 phyB_-5, •) were sown on MS plates containing the indicated concentrations of GA3. Hypocotyl length (A) and GUS activity (B) were determined 7 d after sowing. Values are expressed as means ± 2
se
(n = 12). Time represents days after sowing. Error bars that are not visible are contained within the symbol. MUG, 4-Methylumbelliferyl-β-
d
-glucopyranoside.
Figure 6
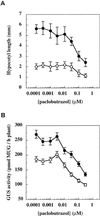
Responses of _ga1_-3 and _ga1_-_3 phyB_-5 seedlings to the GA-biosynthesis-inhibitor paclobutrazol. Seeds of the lines DW150-304 (LFY::GUS, ○) and 304B5 (_LFY::GUS phyB_-5, •) were sown on MS plates containing the indicated concentrations of paclobutrazol. Hypocotyl length (A) and GUS activity (B) were determined 7 d after sowing. Values are expressed as means ± 2
se
(n = 12). Time represents days after sowing. Error bars that are not visible are contained within the symbol. MUG, 4-Methylumbelliferyl-β-
d
-glucopyranoside.
Figure 7
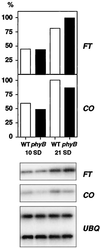
CO and FT RNA expression in phyB mutants. Seedlings of the lines DW150-304 (wild type [WT], white bars) and 304B5 (_phyB_-5, black bars) were grown on MS plates under short days (SD), and harvested during the 8th h of light on the indicated days. Expression was analyzed by RT-PCR (bottom panel) and the signals quantified and normalized using UBQ expression as a control (top panel, arbitrary units).
Similar articles
- Phytochrome B affects responsiveness to gibberellins in Arabidopsis.
Reed JW, Foster KR, Morgan PW, Chory J. Reed JW, et al. Plant Physiol. 1996 Sep;112(1):337-42. doi: 10.1104/pp.112.1.337. Plant Physiol. 1996. PMID: 8819329 Free PMC article. - Effects of gibberellins on seed germination of phytochrome-deficient mutants of Arabidopsis thaliana.
Yang YY, Nagatani A, Zhao YJ, Kang BJ, Kendrick RE, Kamiya Y. Yang YY, et al. Plant Cell Physiol. 1995 Oct;36(7):1205-11. Plant Cell Physiol. 1995. PMID: 8564296 - Spatially distinct regulatory roles for gibberellins in the promotion of flowering of Arabidopsis under long photoperiods.
Porri A, Torti S, Romera-Branchat M, Coupland G. Porri A, et al. Development. 2012 Jun;139(12):2198-209. doi: 10.1242/dev.077164. Epub 2012 May 9. Development. 2012. PMID: 22573618 - Gibberellin as a factor in floral regulatory networks.
Mutasa-Göttgens E, Hedden P. Mutasa-Göttgens E, et al. J Exp Bot. 2009;60(7):1979-89. doi: 10.1093/jxb/erp040. Epub 2009 Mar 5. J Exp Bot. 2009. PMID: 19264752 Review. - Shade Avoidance: Expanding the Color and Hormone Palette.
Fernández-Milmanda GL, Ballaré CL. Fernández-Milmanda GL, et al. Trends Plant Sci. 2021 May;26(5):509-523. doi: 10.1016/j.tplants.2020.12.006. Epub 2021 Jan 16. Trends Plant Sci. 2021. PMID: 33461868 Review.
Cited by
- The cell biology of phytochrome signalling.
Møller SG, Ingles PJ, Whitelam GC. Møller SG, et al. New Phytol. 2002 Jun;154(3):553-590. doi: 10.1046/j.1469-8137.2002.00419.x. New Phytol. 2002. PMID: 33873456 Review. - Phytochrome mediates the external light signal to repress FT orthologs in photoperiodic flowering of rice.
Izawa T, Oikawa T, Sugiyama N, Tanisaka T, Yano M, Shimamoto K. Izawa T, et al. Genes Dev. 2002 Aug 1;16(15):2006-20. doi: 10.1101/gad.999202. Genes Dev. 2002. PMID: 12154129 Free PMC article. - Identification and Validation of New Alleles of FALSIFLORA and COMPOUND INFLORESCENCE Genes Controlling the Number of Branches in Tomato Inflorescence.
Zheng H, Kawabata S. Zheng H, et al. Int J Mol Sci. 2017 Jul 20;18(7):1572. doi: 10.3390/ijms18071572. Int J Mol Sci. 2017. PMID: 28726721 Free PMC article. - EARLY BOLTING IN SHORT DAYS is related to chromatin remodeling factors and regulates flowering in Arabidopsis by repressing FT.
Piñeiro M, Gómez-Mena C, Schaffer R, Martínez-Zapater JM, Coupland G. Piñeiro M, et al. Plant Cell. 2003 Jul;15(7):1552-62. doi: 10.1105/tpc.012153. Plant Cell. 2003. PMID: 12837946 Free PMC article. - Distinct roles of GIGANTEA in promoting flowering and regulating circadian rhythms in Arabidopsis.
Mizoguchi T, Wright L, Fujiwara S, Cremer F, Lee K, Onouchi H, Mouradov A, Fowler S, Kamada H, Putterill J, Coupland G. Mizoguchi T, et al. Plant Cell. 2005 Aug;17(8):2255-70. doi: 10.1105/tpc.105.033464. Epub 2005 Jul 8. Plant Cell. 2005. PMID: 16006578 Free PMC article.
References
- Blázquez MA, Soowal L, Lee I, Weigel D. LEAFY expression and flower initiation in Arabidopsis. Development. 1997;124:3835–3844. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases