Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation - PubMed (original) (raw)
Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation
Y Zhang et al. Genes Dev. 1999.
Abstract
ATP-dependent nucleosome remodeling and core histone acetylation and deacetylation represent mechanisms to alter nucleosome structure. NuRD is a multisubunit complex containing nucleosome remodeling and histone deacetylase activities. The histone deacetylases HDAC1 and HDAC2 and the histone binding proteins RbAp48 and RbAp46 form a core complex shared between NuRD and Sin3-histone deacetylase complexes. The histone deacetylase activity of the core complex is severely compromised. A novel polypeptide highly related to the metastasis-associated protein 1, MTA2, and the methyl-CpG-binding domain-containing protein, MBD3, were found to be subunits of the NuRD complex. MTA2 modulates the enzymatic activity of the histone deacetylase core complex. MBD3 mediates the association of MTA2 with the core histone deacetylase complex. MBD3 does not directly bind methylated DNA but is highly related to MBD2, a polypeptide that binds to methylated DNA and has been reported to possess demethylase activity. MBD2 interacts with the NuRD complex and directs the complex to methylated DNA. NuRD may provide a means of gene silencing by DNA methylation.
Figures
Figure 1
MTA2 and MBD3 are components of the NuRD complex. (A) Silver staining of an SDS-polyacrylamide gel containing purified NuRD (lane 3) and samples derived from different antibody columns, as indicated at top. The identity of each polypeptide was defined by microsequencing and/or Western blot analysis and is indicated at right. The histone deacetylase core complex present in both the Sin3/SAP30 complex and the NuRD complex is indicated with a bracket and denoted as Core. Peptide sequences derived from the bands labeled as MTA2, MBD3a, and MBD3b are presented in Fig. 2. Sequencing of the band labeled as HDAC1* + MBD2 derived from HDAC1 antibody column suggest the presence of both HDAC1 and MBD2. Peptides specific for HDAC1 and MBD2 are VMTVSFHK and GLQGVGPGSNDETLLSAVASALHTSSAPITGQVSAAVEK, respectively. Mass-spectrometric analysis of the three polypeptides between Mi2 and MTA2 found in the conventionally purified NuRD fraction (lane 3) identified these contaminants as HCAP (GenBank accession no. AF020043), SA-1 (GenBank accession no. Z75330), and SB1/DXS423E (GenBank accession no. S78271). (B) Western blot analysis of the samples used in A. The proteins detected are indicated at right.
Figure 2
Sequence analysis of MBD3 and MTA2. (A) Schematic representation of the two forms of MBD2 and MBD3. The open box represent the methyl-CpG binding domain (MBD) initially identified in MeCP2 (also see B). The GenBank accession nos. for MBD2 and MBD3 are AF072242 and AF072247, respectively. The in-frame spliced form MBD3b only contains part of the MBD. (B) Sequences and splicing junctions of the MBD3a and MBD3b. The orange nucleotides that encode 5′ portion of MBD are spliced out in MBD3b. The peptide sequence derived from the band labeled as MBD3a in Fig. 1A is underlined. Other peptides derived from the MBD3 bands of Fig. 1A have perfect match to MBD3: KQEELVQQVR, TMDLPK, GKPDLNTALPVR, and NPGVWLNTTQPLCK. (C) MTA2 is related to the metastasis-associated protein MTA1. Amino acid sequence alignment of human MTA2 (GenBank accession no. AB016591), mouse MTA2 (GenBank accession no. AF159259) with human MTA1 (GenBank accession no. U35113). Sequence alignment was performed using Gap of the GCG program (University of Wisconsin, Madison). Peptide sequences obtained from microsequencing of the band labeled MTA2 in Fig. 1A are underlined. The putative zinc-finger is indicated by green triangles. The putative leucine zipper is indicated by purple dots. The yellow box indicates a potential tyrosine kinase phosphorylation site. Human and mouse MTA2 are 98% identical and are 65% and 63% identical to human MTA1, respectively.
Figure 3
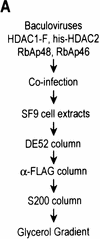
Purification of a recombinant histone deacetylase core complex. (A) Schematic representation of the steps used to purify the histone deacetylase core complex. (B) Silver staining of an SDS-polyacrylamide gel containing the purified core complex. The identities of the major polypeptides and protein size markers are indicated. (C) Western blot analysis of a partially purified HeLa nuclear extracts (lane 1) and the purified core complex (lane 2). The identities of the polypeptides are indicated. (D) Coomassie blue staining of an SDS-polyacrylamide gel containing the purified NuRD complex (lane 1) and individual recombinant NuRD components. Mi2, HDAC1, and MBD3b are purified from baculovirus-infected SF9 cells. MTA2 and RbAps were produced in Escherichia coli. Protein size makers are indicated.
Figure 3
Purification of a recombinant histone deacetylase core complex. (A) Schematic representation of the steps used to purify the histone deacetylase core complex. (B) Silver staining of an SDS-polyacrylamide gel containing the purified core complex. The identities of the major polypeptides and protein size markers are indicated. (C) Western blot analysis of a partially purified HeLa nuclear extracts (lane 1) and the purified core complex (lane 2). The identities of the polypeptides are indicated. (D) Coomassie blue staining of an SDS-polyacrylamide gel containing the purified NuRD complex (lane 1) and individual recombinant NuRD components. Mi2, HDAC1, and MBD3b are purified from baculovirus-infected SF9 cells. MTA2 and RbAps were produced in Escherichia coli. Protein size makers are indicated.
Figure 3
Purification of a recombinant histone deacetylase core complex. (A) Schematic representation of the steps used to purify the histone deacetylase core complex. (B) Silver staining of an SDS-polyacrylamide gel containing the purified core complex. The identities of the major polypeptides and protein size markers are indicated. (C) Western blot analysis of a partially purified HeLa nuclear extracts (lane 1) and the purified core complex (lane 2). The identities of the polypeptides are indicated. (D) Coomassie blue staining of an SDS-polyacrylamide gel containing the purified NuRD complex (lane 1) and individual recombinant NuRD components. Mi2, HDAC1, and MBD3b are purified from baculovirus-infected SF9 cells. MTA2 and RbAps were produced in Escherichia coli. Protein size makers are indicated.
Figure 3
Purification of a recombinant histone deacetylase core complex. (A) Schematic representation of the steps used to purify the histone deacetylase core complex. (B) Silver staining of an SDS-polyacrylamide gel containing the purified core complex. The identities of the major polypeptides and protein size markers are indicated. (C) Western blot analysis of a partially purified HeLa nuclear extracts (lane 1) and the purified core complex (lane 2). The identities of the polypeptides are indicated. (D) Coomassie blue staining of an SDS-polyacrylamide gel containing the purified NuRD complex (lane 1) and individual recombinant NuRD components. Mi2, HDAC1, and MBD3b are purified from baculovirus-infected SF9 cells. MTA2 and RbAps were produced in Escherichia coli. Protein size makers are indicated.
Figure 4
MBD3 interacts with MTA2 and components of the histone deacetylase core complex. (A) GST pull-down assays show that in vitro-translated MTA2 only interacts with MBD3. Equal amounts (5 μl) of in vitro-translated and labeled MTA2 was incubated with 10 μl of glutathione–agarose beads or anti-Flag beads coated with 2 μg of proteins as indicated at the top. After extensive wash, bound proteins were eluted, resolved by SDS-PAGE, and visualized by autoradiography. Ten percent of input was loaded on lane 1. (B) MTA2 directly interacts with MBD3. An experimental procedure similar to A was used except that MTA2 was purified from Escherichia coli (Fig. 3D, lane 3). Bound proteins were eluted, resolved by SDS-PAGE, and visualized by Western blot. (C) MBD3 interacts with components of the core complex in a GST pull-down assay. Assays were performed as in B. Input proteins are indicated at top and are the same as those used in Fig. 3D.
Figure 5
MTA2 is required for the formation of a functional histone deacetylase complex. (A) Histone deacetylase activities of the fractions derived from gel filtration S200 columns. Histone deacetylase core complex as well as core plus MTA2 were purified using the procedure described in Fig. 3A (see Materials and Methods for details). In the histone deacetylation assays using core- and core + MTA2-derived fractions approximately equal Western blot units of HDACs were used. The elution profiles of different size markers are indicated. (B) Silver staining of an SDS-polyacrylamide gel containing the core + MTA2 fractions derived from the S200 gel filtration column analyzed in A. The identities of the polypeptides are indicated and were confirmed by Western blot analysis which is shown in C. (C) Western blot analysis of the fractions shown in B.
Figure 6
MTA2 directs the formation of an enzymatically active histone deacetylase complex. (A) Relative histone deacetylase activities of different coinfections. SF9 cells were infected with different combinations of viruses. The complexes were affinity purified through Flag-tagged HDAC1 using antibodies against Flag. The purified complexes were divided for deacetylase assay shown in A and Western blot analysis shown in B. (+) Presence of the viruses in the coinfection. (B) Western blot analysis of the samples used in histone deacetylation assays shown in A. Antibodies against RbAps cross-reacted with bleached heavy chain in lane 1.
Figure 7
The NuRD complex can be targeted to methylated DNA by MBD2. (A) Gel mobility shift assay shown that the NuRD complex does not bind to methylated DNA directly. The GAM6 probe contains six methyl-CpG sites and was previously described (Nan et al. 1993). Binding reactions were resolved on a 2% agarose gel as described in Materials and Methods. (B) GST pull-down assays demonstrates an interaction between MBD2 and the NuRD complex. The assays were performed as described in Fig. 4B using different GST fusion proteins as indicated at the top. (C) Gel mobility-shift assay shown that the NuRD complex is able to super-shift the DNA–MBD2 binary complex (cf. lanes 2 and 3). The probe used is MeCG11, which contains 27 methyl-CpG sites and was described previously (Ng et al. 1999). Binding reactions were resolved on a 1% agarose gel as described in Materials and Methods.
Similar articles
- MBD3L2 interacts with MBD3 and components of the NuRD complex and can oppose MBD2-MeCP1-mediated methylation silencing.
Jin SG, Jiang CL, Rauch T, Li H, Pfeifer GP. Jin SG, et al. J Biol Chem. 2005 Apr 1;280(13):12700-9. doi: 10.1074/jbc.M413492200. Epub 2005 Jan 27. J Biol Chem. 2005. PMID: 15701600 - MBD3L1 is a transcriptional repressor that interacts with methyl-CpG-binding protein 2 (MBD2) and components of the NuRD complex.
Jiang CL, Jin SG, Pfeifer GP. Jiang CL, et al. J Biol Chem. 2004 Dec 10;279(50):52456-64. doi: 10.1074/jbc.M409149200. Epub 2004 Sep 28. J Biol Chem. 2004. PMID: 15456747 - The MeCP1 complex represses transcription through preferential binding, remodeling, and deacetylating methylated nucleosomes.
Feng Q, Zhang Y. Feng Q, et al. Genes Dev. 2001 Apr 1;15(7):827-32. doi: 10.1101/gad.876201. Genes Dev. 2001. PMID: 11297506 Free PMC article. - Mi-2/NuRD: multiple complexes for many purposes.
Bowen NJ, Fujita N, Kajita M, Wade PA. Bowen NJ, et al. Biochim Biophys Acta. 2004 Mar 15;1677(1-3):52-7. doi: 10.1016/j.bbaexp.2003.10.010. Biochim Biophys Acta. 2004. PMID: 15020045 Review. - The human Mi-2/NuRD complex and gene regulation.
Denslow SA, Wade PA. Denslow SA, et al. Oncogene. 2007 Aug 13;26(37):5433-8. doi: 10.1038/sj.onc.1210611. Oncogene. 2007. PMID: 17694084 Review.
Cited by
- Differential roles for MBD2 and MBD3 at methylated CpG islands, active promoters and binding to exon sequences.
Günther K, Rust M, Leers J, Boettger T, Scharfe M, Jarek M, Bartkuhn M, Renkawitz R. Günther K, et al. Nucleic Acids Res. 2013 Mar 1;41(5):3010-21. doi: 10.1093/nar/gkt035. Epub 2013 Jan 29. Nucleic Acids Res. 2013. PMID: 23361464 Free PMC article. - Epigenetic cancer prevention mechanisms in skin cancer.
Saha K, Hornyak TJ, Eckert RL. Saha K, et al. AAPS J. 2013 Oct;15(4):1064-71. doi: 10.1208/s12248-013-9513-3. Epub 2013 Aug 1. AAPS J. 2013. PMID: 23904153 Free PMC article. Review. - Zic2 is an enhancer-binding factor required for embryonic stem cell specification.
Luo Z, Gao X, Lin C, Smith ER, Marshall SA, Swanson SK, Florens L, Washburn MP, Shilatifard A. Luo Z, et al. Mol Cell. 2015 Feb 19;57(4):685-694. doi: 10.1016/j.molcel.2015.01.007. Mol Cell. 2015. PMID: 25699711 Free PMC article. - Stimulation of histone deacetylase activity by metabolites of intermediary metabolism.
Vogelauer M, Krall AS, McBrian MA, Li JY, Kurdistani SK. Vogelauer M, et al. J Biol Chem. 2012 Sep 14;287(38):32006-16. doi: 10.1074/jbc.M112.362467. Epub 2012 Jul 20. J Biol Chem. 2012. PMID: 22822071 Free PMC article. - Structural insights into the interaction and activation of histone deacetylase 3 by nuclear receptor corepressors.
Codina A, Love JD, Li Y, Lazar MA, Neuhaus D, Schwabe JW. Codina A, et al. Proc Natl Acad Sci U S A. 2005 Apr 26;102(17):6009-14. doi: 10.1073/pnas.0500299102. Epub 2005 Apr 18. Proc Natl Acad Sci U S A. 2005. PMID: 15837933 Free PMC article.
References
- Airio A, Pukkala E, Isomaki H. Elevated cancer incidence in patients with dermatomyositis: A population based study. J Rheumatol. 1995;22:1300–1303. - PubMed
- Amati B, Land H. Myc-Max-Mad: A transcription factor network controlling cell cycle progression, differentiation and death. Curr Opin Genet Dev. 1994;4:102–108. - PubMed
- Armstrong JA, Bieker JJ, Emerson BM. A SWI/SNF-related chromatin remodeling complex, E-RC1, is required for tissue-specific transcriptional regulation by EKLF in vitro. Cell. 1998;95:93–104. - PubMed
- Avitahl N, Winandy S, Friedrich C, Jones B, Ge Y, Georgopoulos K. Ikaros sets thresholds for T cell activation and regulates chromosome propagation. Immunity. 1999;10:333–343. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous