Inhibition of T-cell responsiveness by nasal peptide administration: influence of the thymus and differential recovery of T-cell-dependent functions - PubMed (original) (raw)
Inhibition of T-cell responsiveness by nasal peptide administration: influence of the thymus and differential recovery of T-cell-dependent functions
B Metzler et al. Immunology. 1999 Jun.
Abstract
We have previously demonstrated that intranasal (i.n.) administration of the major encephalitogenic peptide, Ac1-9 of myelin basic protein (MBP), inhibited T-cell responsiveness in vitro and induced tolerance in the H-2u mouse model of experimental autoimmune encephalomyelitis (EAE). The peptide analogue Ac1-9[4Y] with high-affinity binding to the I-Au major histocompatibility complex (MHC) class II molecule was the most effective tolerogen. Here, we show that mice pretreated with 4Y i.n. and primed with myelin had strongly reduced levels of anti-MBP immunoglobulin G2a (IgG2a) and IgG1, demonstrating that both T helper 1 (Th1) and Th2 functions were inhibited in vivo. Since peptide administered i.n. was shown to be functionally relevant in the thymus, the time interval between 4Y i.n. and subsequent priming was varied in euthymic and adult thymectomized (ATx) mice, to examine the duration of in vitro cell unresponsiveness in the presence or absence of a thymus. For intervals of 1-6 weeks, inhibition of T-cell proliferation was virtually complete in both euthymic and ATx mice. From 8 weeks onwards, responsiveness slowly recovered in euthymic but not in ATx mice. With an interval of 16 weeks, substantial recovery of T-cell responsiveness in vitro in euthymic mice was reflected by a low degree of protection from EAE in vivo. By contrast, anti-MBP IgG2a and IgG1 antibody responses were still significantly reduced. These findings suggest that T-cell unresponsiveness by peptide i.n. represents a thymus-independent, peripheral phenomenon, the reversal of which is confined to new T cells being exported from the thymus. As observed for EAE and antibody responses, the kinetics of recovery may vary for different effector functions.
Figures
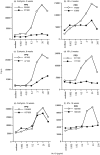
Figure 1
Establishment and duration of T-cell unresponsiveness in euthymic and adult thymectomized (ATx) mice. Atx mice (b, d, f) or euthymic littermates (a, c, e) received a single 100 μg dose of Ac1–9[4Y] i.n. (•) or PBS (○). Mice were subsequently primed with 50 μg Ac1–9/CFA at one of the indicated time-points after 4Y i.n.; 2 weeks (a, b), 8 weeks (c, d), or 16 weeks (e, f). Ten days later, primed lymphocytes were tested for proliferative responses to Ac1–9 in vitro. Each symbol represents the arithmetic means of triplicate samples, standard deviations were less than 20%. Lymphocyte samples were either derived from individual mice, or pooled from two mice. Each plot shows proliferation data of a representative set of lymphocyte samples with comparable positive (PPD) and negative (medium only) control responses. All samples (three to six independent cell samples per group in two or three experiments per time-point) with comparable negative and positive controls showed similar differences in proliferation.
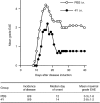
Figure 2
Partial reversal of protection from EAE 16 weeks after 4Y i.n. Groups of nine mice received a single 100 μg dose of Ac1–9[4Y] i.n. (•) or PBS (○).Sixteen weeks later, EAE was induced with 1 mg SCH/CFA.
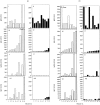
Figure 3
Effect of 4Y i.n. on anti-MBP antibody responses. Groups of nine or 10 mice received a single intranasal dose of 100 μg Ac1–9[4Y] (ii, iv, vi, viii; filled bars) or PBS (i, iii, v, vii; open bars). One week (a) or 16 weeks (b) later, all were primed with 1 mg SCH/CFA. After 3 weeks, serum samples from individual mice were assayed by ELISA for anti-MBP antibody concentrations: IgM (i, ii), total IgG (iii, iv), IgG2a (v, vi), and IgG1(vii, vii). Results are expressed in arbitrary units per ml serum as determined from standard curves with positive sera. One unit corresponds to ≈50% maximum absorbance obtained with the standard titration at 1/250 or 1/500 dilution. Sera are numbered in order of increasing concentrations of total IgG. The same numbers apply to all other isotype data plots.
Similar articles
- Inhibition of experimental autoimmune encephalomyelitis in Lewis rats by nasal administration of encephalitogenic MBP peptides: synergistic effects of MBP 68-86 and 87-99.
Liu JQ, Bai XF, Shi FD, Xiao BG, Li HL, Levi M, Mustafa M, Wahren B, Link H. Liu JQ, et al. Int Immunol. 1998 Aug;10(8):1139-48. doi: 10.1093/intimm/10.8.1139. Int Immunol. 1998. PMID: 9723700 - Mechanisms of mouse T lymphocyte-induced suppression of the IgG2ab allotype and T lymphocyte tolerance to IgG2ab.
Majlessi L, Bordenave G. Majlessi L, et al. Arch Immunol Ther Exp (Warsz). 2001;49(6):407-15. Arch Immunol Ther Exp (Warsz). 2001. PMID: 11814234 Review. - Shifting T-cell activation thresholds in autoimmunity and determinant spreading.
Lehmann PV, Targoni OS, Forsthuber TG. Lehmann PV, et al. Immunol Rev. 1998 Aug;164:53-61. doi: 10.1111/j.1600-065x.1998.tb01207.x. Immunol Rev. 1998. PMID: 9795763 Review.
Cited by
- Advancement of antigen-specific immunotherapy: knowledge transfer between allergy and autoimmunity.
Richardson N, Wraith DC. Richardson N, et al. Immunother Adv. 2021 May 22;1(1):ltab009. doi: 10.1093/immadv/ltab009. eCollection 2021 Jan. Immunother Adv. 2021. PMID: 35919740 Free PMC article. Review. - Antigen-Specific Immunotherapy for Treatment of Autoimmune Liver Diseases.
Richardson N, Ng STH, Wraith DC. Richardson N, et al. Front Immunol. 2020 Jul 21;11:1586. doi: 10.3389/fimmu.2020.01586. eCollection 2020. Front Immunol. 2020. PMID: 32793226 Free PMC article. Review. - Preclinical development and first-in-human study of ATX-MS-1467 for immunotherapy of MS.
Streeter HB, Rigden R, Martin KF, Scolding NJ, Wraith DC. Streeter HB, et al. Neurol Neuroimmunol Neuroinflamm. 2015 Mar 12;2(3):e93. doi: 10.1212/NXI.0000000000000093. eCollection 2015 Jun. Neurol Neuroimmunol Neuroinflamm. 2015. PMID: 25798453 Free PMC article. - PLGA, PLGA-TMC and TMC-TPP nanoparticles differentially modulate the outcome of nasal vaccination by inducing tolerance or enhancing humoral immunity.
Keijzer C, Slütter B, van der Zee R, Jiskoot W, van Eden W, Broere F. Keijzer C, et al. PLoS One. 2011;6(11):e26684. doi: 10.1371/journal.pone.0026684. Epub 2011 Nov 2. PLoS One. 2011. PMID: 22073184 Free PMC article. - Long term immunologic consequences of experimental stroke and mucosal tolerance.
Gee JM, Zierath D, Hadwin J, Savos A, Kalil A, Thullbery M, Becker KJ. Gee JM, et al. Exp Transl Stroke Med. 2009 Oct 21;1:3. doi: 10.1186/2040-7378-1-3. Exp Transl Stroke Med. 2009. PMID: 20142990 Free PMC article.
References
- Metzler B, Wraith DC. Inhibition of experimental autoimmune encephalomyelitis by inhalation but not oral administration of the encephalitogenic peptide: influence of MHC binding affinity. Intern Immunol. 1993;5:1159. - PubMed
- Metzler B, Wraith DC. Mucosal tolerance in a murine model of experimental autoimmune encephalomyelitis. Ann N Y Acad Sci. 1996;778:228. - PubMed
- Staines NA, Harper N, Ward FJ, Malmstrom V, Holmdahl R, Bansal S. Mucosal tolerance and suppression of collagen-induced arthritis (CIA) induced by nasal inhalation of synthetic peptide 184 of bovine type II collagen (CII) expressing a dominant T cell epitope. Clin Exp Immunol. 1996;103:368. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous