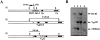B cell antigen receptor specificity and surface density together determine B-1 versus B-2 cell development - PubMed (original) (raw)
B cell antigen receptor specificity and surface density together determine B-1 versus B-2 cell development
K P Lam et al. J Exp Med. 1999.
Abstract
Mice expressing the immunoglobulin (Ig) heavy (H) chain variable (V) region from a rearranged V(H)12 gene inserted into the IgH locus generate predominantly B-1 cells, whereas expression of two other V(H) region transgenes (V(H)B1-8 and V(H)glD42) leads to the almost exclusive generation of conventional, or B-2, cells. To determine the developmental potential of B cells bearing two distinct B cell antigen receptors (BCRs), one favoring B-1 and the other favoring B-2 cell development, we crossed V(H)12 insertion mice with mice bearing either V(H)B1-8 or V(H)glD42. B cells coexpressing V(H)12 and one of the other V(H) genes are readily detected in the double IgH insertion mice, and are of the B-2 phenotype. In mice coexpressing V(H)12, V(H)B1-8 and a transgenic kappa chain able to pair with both H chains, double H chain-expressing B-2 cells, and B-1 cells that have lost V(H)B1-8 are generated, whereas V(H)B1-8 single producers are undetectable. These data suggest that B-1 but not B-2 cells are selected by antigenic stimuli in whose delivery BCR specificity and surface density are of critical importance.
Figures
Figure 1
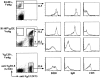
Phenotype of splenic B cells in (A) wild-type, glD42i/+, VH12f/+, and VH12f/glD42i; and (B) B1-8f/+, VH12f/+, and VH12f/B1-8f IgH insertion mice. Spleen cells obtained from wild-type and various Ig H chain insertion mice were stained with fluorochrome-conjugated allotypic (A, top) and idiotypic (B, top) antibodies as well as antibodies that recognize various cell surface markers used to define B-1 and B-2 cells. Numbers indicate percentage of total lymphocytes in the top panels and percentage of total B220+ cells in the others.
Figure 2
Association of the Vκ4 L chain with the VH12 and VHB1-8 H chains. Bone marrow cells from wild-type, VH12f/+, B1-8f/+, or VH12f/B1-8f mice with or without the Vκ4 L chain transgene were stained with anti-B220, anti-CD43, and anti-IgM mAbs. The figure depicts the surface IgM− cells and the boxed area indicates the pre-B cell compartment in the bone marrow. Numbers indicate percentage of total lymphocytes.
Figure 4
B cell populations found in the peritoneal cavity of B1-8f/+, VH12f/+, and VH12f/B1-8f mice. Peritoneal cavity cells of the various single and double IgH insertion mice were stained with antiidiotypic Ac146 and anti-IgM (top) and Ac146 and 5C5 (bottom) mAbs. Numbers indicate percentage of total lymphocytes.
Figure 3
B cells coexpressing VH12, Vκ4, and VHB1-8, Vκ4 assume a conventional B cell phenotype. Phenotype of the various splenic B cell populations found in B1-8f/+, Vκ4tg; VH12f/+, Vκ4tg; and VH12f/B1-8f, Vκ4tg mice. Cells were stained with antiidiotypic Ac146 and 5C5 mAbs, and with anti-B220, anti-IgD, and anti-CD5 mAbs. Numbers indicate percentage of total splenic lymphocytes.
Figure 5
Single VH12, Vκ4–expressing B cells in VH12f/B1-8f, Vκ4tg mice have lost the targeted B1-8f H chain allele. (A) Structures of the wild-type IgH locus 1, the targeted locus bearing the B1-8 VDJ 2, and the targeted locus bearing the VH12 VDJ 3 are shown together with the size of the respective restriction fragments. Genomic DNA was digested with BamHI and hybridized with the indicated probe. Maps are not drawn to scale. (B) Southern blot analysis of DNA from livers of B1-8f/+, Vκ4tg (lane 1); VH12f/+, Vκ4tg (lane 2); and VH12f/B1-8f, Vκ4tg (lane 3) mice; and FACS® sorted 5C5+Ac146− splenic B cells of VH12f/B1-8f, Vκ4tg mice (lane 4).
Similar articles
- Pre-B cell receptor-mediated selection of pre-B cells synthesizing functional mu heavy chains.
Kline GH, Hartwell L, Beck-Engeser GB, Keyna U, Zaharevitz S, Klinman NR, Jäck HM. Kline GH, et al. J Immunol. 1998 Aug 15;161(4):1608-18. J Immunol. 1998. PMID: 9712022 - Progressive surface B cell antigen receptor down-regulation accompanies efficient development of antinuclear antigen B cells to mature, follicular phenotype.
Heltemes-Harris L, Liu X, Manser T. Heltemes-Harris L, et al. J Immunol. 2004 Jan 15;172(2):823-33. doi: 10.4049/jimmunol.172.2.823. J Immunol. 2004. PMID: 14707052 - Frequency and characterization of phenotypic Ig heavy chain allelically included IgM-expressing B cells in mice.
Barreto V, Cumano A. Barreto V, et al. J Immunol. 2000 Jan 15;164(2):893-9. doi: 10.4049/jimmunol.164.2.893. J Immunol. 2000. PMID: 10623837 - Repertoire selection by pre-B-cell receptors and B-cell receptors, and genetic control of B-cell development from immature to mature B cells.
Melchers F, ten Boekel E, Seidl T, Kong XC, Yamagami T, Onishi K, Shimizu T, Rolink AG, Andersson J. Melchers F, et al. Immunol Rev. 2000 Jun;175:33-46. Immunol Rev. 2000. PMID: 10933589 Review. - Selection in the mature B cell repertoire.
Martin F, Kearney JF. Martin F, et al. Curr Top Microbiol Immunol. 2000;252:97-105. doi: 10.1007/978-3-642-57284-5_11. Curr Top Microbiol Immunol. 2000. PMID: 11125496 Review. No abstract available.
Cited by
- B-1 lymphocytes in adipose tissue as innate modulators of inflammation linked to cardiometabolic disease.
Meher AK, McNamara CA. Meher AK, et al. Immunol Rev. 2024 Jul;324(1):95-103. doi: 10.1111/imr.13342. Epub 2024 May 15. Immunol Rev. 2024. PMID: 38747455 Free PMC article. Review. - CK2β-regulated signaling controls B cell differentiation and function.
Quotti Tubi L, Mandato E, Canovas Nunes S, Arjomand A, Zaffino F, Manni S, Casellato A, Macaccaro P, Vitulo N, Zumerle S, Filhol O, Boldyreff B, Siebel CW, Viola A, Valle G, Mainoldi F, Casola S, Cancila V, Gulino A, Tripodo C, Pizzi M, Dei Tos AP, Trentin L, Semenzato G, Piazza F. Quotti Tubi L, et al. Front Immunol. 2023 Jan 11;13:959138. doi: 10.3389/fimmu.2022.959138. eCollection 2022. Front Immunol. 2023. PMID: 36713383 Free PMC article. - H3K36 methyltransferase NSD1 is essential for normal B1 and B2 cell development and germinal center formation.
Zhai S, Cao M, Zhou H, Zhu H, Xu T, Wang Y, Wang X, Cai Z. Zhai S, et al. Front Immunol. 2022 Nov 30;13:959021. doi: 10.3389/fimmu.2022.959021. eCollection 2022. Front Immunol. 2022. PMID: 36532012 Free PMC article. - Replacement of TCR Dβ With Immunoglobulin DH DSP2.3 Imposes a Tyrosine-Enriched TCR Repertoire and Adversely Affects T Cell Development.
Levinson M, Khass M, Burrows PD, Schroeder HW Jr. Levinson M, et al. Front Immunol. 2020 Sep 29;11:573413. doi: 10.3389/fimmu.2020.573413. eCollection 2020. Front Immunol. 2020. PMID: 33133088 Free PMC article. - B-1a cells acquire their unique characteristics by bypassing the pre-BCR selection stage.
Wong JB, Hewitt SL, Heltemes-Harris LM, Mandal M, Johnson K, Rajewsky K, Koralov SB, Clark MR, Farrar MA, Skok JA. Wong JB, et al. Nat Commun. 2019 Oct 18;10(1):4768. doi: 10.1038/s41467-019-12824-z. Nat Commun. 2019. PMID: 31628339 Free PMC article.
References
- Herzenberg L.A., Stall A.M., Lalor P.A., Sidman C., Moore W.A., Parks D., Herzenberg L.A. The Ly-1 B cell lineage. Immunol. Rev. 1986;93:81–102. - PubMed
- Kantor A.B., Herzenberg L.A. Origin of murine B cell lineages. Annu. Rev. Immunol. 1993;11:501–538. - PubMed
- Lalor P.A., Morahan G. The peritoneal Ly-1 (CD5) B cell repertoire is unique among murine B cell repertoires. Eur. J. Immunol. 1990;20:485–492. - PubMed
- Pennell C.A., Arnold L.W., Haughton G., Clarke S.H. Restricted Ig variable region gene expression among Ly-1+ B cell lymphomas. J. Immunol. 1988;141:2788–2796. - PubMed
- Haughton G., Arnold L.W., Whitmore A.C., Clarke S.H. B-1 cells are made, not born. Immunol. Today. 1993;14:84–87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases