Expression of CD28 and CD86 by human eosinophils and role in the secretion of type 1 cytokines (interleukin 2 and interferon gamma): inhibition by immunoglobulin a complexes - PubMed (original) (raw)
Expression of CD28 and CD86 by human eosinophils and role in the secretion of type 1 cytokines (interleukin 2 and interferon gamma): inhibition by immunoglobulin a complexes
G Woerly et al. J Exp Med. 1999.
Abstract
Eosinophils are the source of various immunoregulatory cytokines, but the membrane molecules involved in their secretion have not been clearly identified. Here we show that peripheral blood eosinophils from hypereosinophilic patients could express membrane CD86 but not CD80. The T cell costimulatory molecule CD28 is also detected on the eosinophil surface. CD28 ligation but not CD86 ligation resulted in interleukin (IL)-2 and interferon (IFN)-gamma secretion by eosinophils, whereas IL-4, IL-5, and IL-10 were not detected. In contrast to T cells requiring two signals for effective stimulation, CD28 ligation alone was sufficient for optimal eosinophil activation. Eosinophil-derived IL-2 and IFN-gamma were biologically active, as supernatants from anti-CD28-treated cells were able to induce CTLL-2 proliferation and major histocompatibility complex class II expression on the colon carcinoma cell line Colo 205, respectively. Addition of secretory immunoglobulin (Ig)A-anti-IgA complexes, which could induce the release of IL-10, very significantly inhibited both CD28-mediated IL-2 and IFN-gamma release. These results suggest that the release of type 1 (IFN-gamma and IL-2) versus type 2 cytokines by eosinophils is not only differential but also dependent on cross-regulatory signals. They confirm that through activation of costimulatory molecules, eosinophils could function as an immunoregulatory cell involved in the release of both type 1 and type 2 cytokines.
Figures
Figure 1
Intracellular production of IL-2, IFN-γ, and IL-10 by human eosinophils. Freshly purified eosinophils (purity >98%) were analyzed by direct immunofluorescence flow cytometry (A) and immunocytochemistry (B). (A) After fixation and permeabilization, the cells were stained with FITC-conjugated anti–IL-2, anti–IFN-γ, or anti–IL-10 (bold line) or isotype-matched Ab (dashed line), as described in Materials and Methods. Cell fluorescence was measured using a FACSCalibur™ equipped with CellQuest software, and thresholds were set according to the isotype-matched controls. A total of 104 cells were usually acquired. (B) Cytospins of eosinophil preparation were incubated with anti–IL-2, anti–IFN-γ, anti–IL-10 mAb or isotype-matched Ab (inset), and the staining was revealed using the APAAP detection system and New Fuchsin coloration. Cells were counterstained with Mayer's hematoxylin (original magnification: ×100).
Figure 2
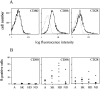
Expression of costimulatory molecules of the CD28/B7 family by human eosinophils. (A) Representative profiles of anti-CD80, anti-CD86, and anti-CD28 mAb binding to freshly purified human eosinophils. Cells were stained with FITC-conjugated anti-CD80, anti-CD86, or anti-CD28 mAb (bold line) or isotype-matched control Ab (dotted line). Samples were analyzed by flow cytometry using a FACSCalibur™ equipped with CellQuest software. A total of 104 cells were usually acquired. (B) Variable expression of CD86 and CD28 by individual patients. Data are presented as percentage of positive cells from 17 eosinophilic patients (Table , donors 1–17) and 5 normal donors (Table , donors 22–26). The donors were classified as follows: A, allergy; SK, skin diseases; HD, hematological disorders; ND, normal donors. The percentage of positive cells was calculated by subtracting the isotype control from the specific signal.
Figure 3
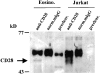
CD28 expression in human eosinophils. Western blot analysis of freshly purified eosinophils (Eosino., 2.5 × 107) and Jurkat T cells (2 × 107) after sequential immunoprecipitation with unconjugated Sepharose beads (preclear.), mouse IgG control Ab (norm.mIgG), and anti-CD28 mAb (anti-CD28 [9.3 mAb]). Material was run on 8% SDS-PAGE and transferred to nitrocellulose. Membrane was probed with anti-CD28 Ab.
Figure 4
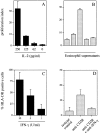
Release of IL-2 and IFN-γ by eosinophils after stimulation with anti-CD28 mAb. For stimulation, wells of a 24-well plate were first coated with 40 μg/ml anti–mouse IgG F(ab′)2, followed by 10 μg/ml anti-CD28, anti-CD86, or isotype mouse IgG1 control Ab. After washing, freshly purified eosinophils (2 × 106 in 1 ml) were added per well. After 18 h, supernatants were harvested and analyzed by ELISA. Grey bars, immobilized anti-CD28 mAb–stimulated eosinophils; black bars, immobilized anti-CD86 mAb–stimulated eosinophils; hatched bars, mouse IgG1 control. Results were obtained from six individual patients (Table , donors 2, 8, 11, 16, 19, and 21).
Figure 5
Biological activity of IL-2 and IFN-γ released by CD28-activated eosinophils. (A and B) Detection of the proliferative activity of IL-2 released by eosinophils on an IL-2–dependent cell line. Supernatants collected from eosinophils (2 × 106/ml) stimulated with immobilized anti-CD28 mAb or isotype control Ab for 18 h were able to sustain the IL-2–dependent growth of the mouse cytotoxic T cell line CTLL-2 (American Type Culture Collection). The CTLL-2 cells were cultured in the presence of rhIL-2 (A) or eosinophil supernatants (50% dilution; B) for 24 h. Proliferation was measured by [3H]thymidine incorporation. Each supernatant obtained from individual patients (Table , donors 2, 11, and 16) was assayed in duplicate. Results are expressed as proliferation index ± SD, according to the following formula: proliferation index = dpm (eosinophils stimulated with anti-CD28 mAb)/dpm (eosinophils stimulated with isotype control mAb). An index >3 was considered significant. (C and D) Supernatants collected from eosinophils (2 × 106/ml) stimulated with cross-linked anti-CD28 mAb or isotype control Ab for 18 h were assayed for IFN-γ–induced MHC class II expression on Colo 205 cells. The Colo 205 cells were cultured in the presence of rhIFN-γ (C) or eosinophil supernatants (20% dilution; D). After 48 h, cells were harvested and stained with a PE-conjugated anti–HLA-DR mAb or an isotype-matched Ab and examined by flow cytometry. Specificity of the bioassay was controlled by adding a neutralizing anti–IFN-γ mAb to the culture. Cell fluorescence was measured using a FACSCalibur™ flow cytometer, and data were analyzed using CellQuest software. Data are presented as mean ± SD from three different experiments (Table , donors 2, 8, and 16).
Figure 6
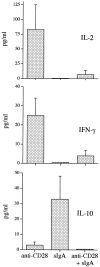
Reciprocal effect of IgA–anti-IgA complexes and CD28 on IL-10, IL-2, and IFN-γ release. Purified eosinophils (2 × 106/ml) were stimulated with either immobilized anti-CD28 mAb, IgA–anti-IgA, or IgA followed by anti-IgA together with immobilized anti-CD28 as described in Materials and Methods. After 18 h, supernatants were harvested and analyzed by ELISA for cytokine content. Data were obtained from three different patients (Table , donors 18, 20, and 21) and are presented as mean ± SEM.
Similar articles
- Both CD28 ligands CD80 (B7-1) and CD86 (B7-2) activate phosphatidylinositol 3-kinase, and wortmannin reveals heterogeneity in the regulation of T cell IL-2 secretion.
Ueda Y, Levine BL, Huang ML, Freeman GJ, Nadler LM, June CH, Ward SG. Ueda Y, et al. Int Immunol. 1995 Jun;7(6):957-66. doi: 10.1093/intimm/7.6.957. Int Immunol. 1995. PMID: 7577804 - Endothelial cells modify the costimulatory capacity of transmigrating leukocytes and promote CD28-mediated CD4(+) T cell alloactivation.
Denton MD, Geehan CS, Alexander SI, Sayegh MH, Briscoe DM. Denton MD, et al. J Exp Med. 1999 Aug 16;190(4):555-66. doi: 10.1084/jem.190.4.555. J Exp Med. 1999. PMID: 10449526 Free PMC article. - CD28 ligands CD80 (B7-1) and CD86 (B7-2) induce long-term autocrine growth of CD4+ T cells and induce similar patterns of cytokine secretion in vitro.
Levine BL, Ueda Y, Craighead N, Huang ML, June CH. Levine BL, et al. Int Immunol. 1995 Jun;7(6):891-904. doi: 10.1093/intimm/7.6.891. Int Immunol. 1995. PMID: 7577797 - Human neutrophil-expressed CD28 interacts with macrophage B7 to induce phosphatidylinositol 3-kinase-dependent IFN-gamma secretion and restriction of Leishmania growth.
Venuprasad K, Banerjee PP, Chattopadhyay S, Sharma S, Pal S, Parab PB, Mitra D, Saha B. Venuprasad K, et al. J Immunol. 2002 Jul 15;169(2):920-8. doi: 10.4049/jimmunol.169.2.920. J Immunol. 2002. PMID: 12097397 - Cytokines: is there biological meaning?
Mosmann TR. Mosmann TR. Curr Opin Immunol. 1991 Jun;3(3):311-4. doi: 10.1016/0952-7915(91)90029-z. Curr Opin Immunol. 1991. PMID: 1910608 Review.
Cited by
- CD69 Signaling in Eosinophils Induces IL-10 Production and Apoptosis via the Erk1/2 and JNK Pathways, Respectively.
Bui DV, Nguyen LM, Kanda A, Chu HH, Thi Le NK, Yun Y, Kobayashi Y, Suzuki K, Mitani A, Shimamura A, Fukui K, Sawada S, Dombrowicz D, Iwai H. Bui DV, et al. Biomolecules. 2024 Mar 18;14(3):360. doi: 10.3390/biom14030360. Biomolecules. 2024. PMID: 38540778 Free PMC article. - The bidirectional immune crosstalk in metabolic dysfunction-associated steatotic liver disease.
Sawada K, Chung H, Softic S, Moreno-Fernandez ME, Divanovic S. Sawada K, et al. Cell Metab. 2023 Nov 7;35(11):1852-1871. doi: 10.1016/j.cmet.2023.10.009. Cell Metab. 2023. PMID: 37939656 Free PMC article. Review. - Eosinophils as potential biomarkers in respiratory viral infections.
Macchia I, La Sorsa V, Urbani F, Moretti S, Antonucci C, Afferni C, Schiavoni G. Macchia I, et al. Front Immunol. 2023 Jul 6;14:1170035. doi: 10.3389/fimmu.2023.1170035. eCollection 2023. Front Immunol. 2023. PMID: 37483591 Free PMC article. Review. - Eosinophil-lymphocyte interactions in the tumor microenvironment and cancer immunotherapy.
Grisaru-Tal S, Rothenberg ME, Munitz A. Grisaru-Tal S, et al. Nat Immunol. 2022 Sep;23(9):1309-1316. doi: 10.1038/s41590-022-01291-2. Epub 2022 Aug 24. Nat Immunol. 2022. PMID: 36002647 Free PMC article. Review. - Blood Eosinophils Are Associated with Efficacy of Targeted Therapy in Patients with Advanced Melanoma.
Wendlinger S, Wohlfarth J, Kreft S, Siedel C, Kilian T, Dischinger U, Heppt MV, Wistuba-Hamprecht K, Meier F, Goebeler M, Schadendorf D, Gesierich A, Kosnopfel C, Schilling B. Wendlinger S, et al. Cancers (Basel). 2022 May 4;14(9):2294. doi: 10.3390/cancers14092294. Cancers (Basel). 2022. PMID: 35565423 Free PMC article.
References
- Weller P.F. Updates on cells and cytokineshuman eosinophils. J. Allergy Clin. Immunol. 1997;100:283–287. - PubMed
- Nonaka M., Nonaka R., Woolley K., Adelroth E., Miura K., Ohkawara Y., Glibetic M., Nakano K., O'Byrne P., Dolovich J., Jordana M. Distinct immunohistochemical localization of IL-4 in human inflamed airway tissues. IL-4 is localized to eosinophils in vivo and is released by peripheral blood eosinophils. J. Immunol. 1995;155:3234–3244. - PubMed
- Moqbel R., Yin S., Barkans J., Newman T.M., Kimmitt P., Wakelin M., Taborda-Barata L., Meng Q., Corrigan C.J., Durham S.R., Kay A.B. Identification of messenger RNA for IL-4 in human eosinophils with granule localization and release of the translated product. J. Immunol. 1995;155:4939–4947. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous