The direction of transport through the nuclear pore can be inverted - PubMed (original) (raw)
The direction of transport through the nuclear pore can be inverted
M V Nachury et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Transport of macromolecules across the nuclear envelope is an active process that depends on soluble factors including the GTPase Ran. Ran-GTP is predominantly located in the nucleus and has been shown to regulate cargo binding and release of import and export receptors in their respective target compartments. Recently, it was shown that transport of receptor-cargo complexes across the nuclear pore complex (NPC) does not depend on GTP-hydrolysis by Ran; however, the mechanism of translocation is still poorly understood. Here, we show that the direction of transport through the NPC can be inverted in the presence of high concentrations of cytoplasmic Ran-GTP. Under these conditions, two different classes of export cargoes are transported into the nucleus in the absence of GTP hydrolysis. The inverted transport is very rapid and can be blocked by known inhibitors of nuclear protein export. These results suggest that the NPC functions as a facilitated transport channel, allowing the selective translocation of receptor-cargo complexes. We conclude that the directionality of nucleocytoplasmic transport is determined mainly by the compartmentalized distribution of Ran-GTP.
Figures
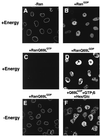
Figure 1
RanQ69L supports import in vitro. Nuclear import of fluorescein-labeled IBB-βGal was assayed in permeabilized cells for 30 min in the presence of 1 μM importin β and buffer alone (A), 2.5 μM Ran-GDP (B), 2.5 μM RanQ69L-GTP (C), or 2.5 μM RanQ69L-GDP (D–F). An energy-regenerating system was present in the reactions shown in A–D. In the reaction shown in F, GTPγS was added to a final concentration of 2.5 mM, and an energy-depleting system was present during the reaction in the form of 0.4 units/μl hexokinase and 10 mM glucose (Hex/Glc). To ensure that no nucleotide triphosphates were present at the beginning of the import reactions, permeabilized cells were also pretreated with hexokinase and glucose before the import reactions in the experiments shown in E and F. Note that the energy-regeneration system used in D could be replaced by 2.5 mM GTP without any decrease in import activity (not shown). The addition of hexokinase/glucose to such a reaction completely abolishes import (not shown), thus showing that this system efficiently depletes GTP in digitonin-permeabilized. The final concentration of the fluorescent import substrate IBB-βGal was 800 nM. Nuclei were scanned with a confocal fluorescence microscope (63× oil objective).
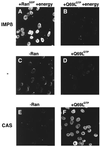
Figure 2
Importin α is translocated into the nucleus in the presence of CAS and Ran-GTP. Nuclear transport of importin α was assayed in digitonin-permeabilized cells for 30 min at 22°C in the presence of recombinant transport factors. A preformed importin α⋅β complex was mixed with either Ran-GDP (A) or RanQ69L-GTP (B) in the presence of an energy-regeneration system before addition to the cells. Similarly, importin α was mixed with buffer alone (C), RanQ69L-GTP alone (D), CAS alone (E), or CAS and RanQ69L-GTP (F). Before addition to the cells, mixes were incubated on ice for 3 min to allow for complex formation. ATP, GTP, and an energy-regeneration system were not added in reactions C–F. Cells were fixed immediately with paraformaldehyde, and importin α was detected by secondary immunofluorescence (35). The following final concentrations of factors were used in these assays: 1 μM importin α, 1 μM importin β, 2 μM CAS, and 2.5 μM Ran. Nuclei were scanned with a confocal fluorescence microscope (63× oil objective).
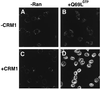
Figure 3
BSA-NES is translocated into the nucleus in the presence of CRM1 and Ran-GTP. Fluorescein-labeled BSA-NES was mixed with buffer alone (A), RanQ69L-GTP alone (B), CRM1 alone (C), or CRM1 and RanQ69L-GTP (D). Mixes were incubated for 3 min on ice to allow for complex formation before addition to digitonin-permeabilized cells. The transport reactions were allowed to proceed at 22°C for 20 min, at which point the cells were immediately fixed on ice with paraformaldehyde. Reactions were performed in the absence of ATP, GTP, or an energy-regeneration system. The following final concentrations of factors were used in these assays: 2.5 μM RanQ69L-GTP, 2 μM CRM1, and 250 nM BSA-NES. Nuclei were scanned with a confocal fluorescence microscope (63× oil objective).
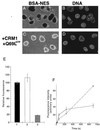
Figure 4
BSA-NES equilibrates between cytoplasmic and nuclear compartments in the presence of CRM1 and RanQ69L-GTP. Cells grown on coverslips were permeabilized with digitonin and washed in the presence of the DNA-stain 4′,6-diamidino-2-phenylindole. Coverslips were then inverted onto a droplet containing either 250 nM fluorescein-labeled BSA-NES alone (A and B) or together with 2.5 μM RanQ69L-GTP and 2 μM CRM1 (C and D). The localization of the fluorescein-labeled BSA-NES was followed by laser scanning confocal microscopy (63× oil objective) through the FITC channel (A and C), whereas the nuclear DNA was visualized through the UV channel (B and D). Images were recorded after 10 min of incubation at 22°C. Note that the levels of intranuclear fluorescence in C are similar to the level in the surrounding solution, whereas no fluorescent signal can be seen in the nuclei shown in A. (E) The extracellular fluorescence (bar 1) for the reactions shown in A and C was compared with the intranuclear fluorescence shown in C (bar 2) and with that shown in A (bar 3). More than 50 cells from five different fields were analyzed for each data point. The error bars correspond to the standard deviations between fields. (F) Kinetics of reversed NES export (solid line) and Xenopus extract-driven IBB import (dashed line) were estimated by fixing cells at 15 s, 1 min, 4 min, and 15 min. Use of recombinant factors instead of Xenopus extracts for the import of IBB-βGal resulted in a similar, continuous accumulation of substrate over time (data not shown). Because the two substrates differ in the number of conjugated fluorophores, the fluorescence intensities cannot be compared directly between the two assays. To estimate the rate of inverted export, we assumed that the average nuclear diameter is 10 μm (a perfectly round HeLa cell nucleus has therefore a volume of 12.5 pl), that a HeLa cell nucleus contains ≈3,000 NPCs, that at short time points no reexit of the substrate has occurred, and that, at equilibrium, the substrate concentration inside and outside the nuclei was 0.3 μM. Because, at 2 min, the intranuclear intensity is about 50% of that at equilibration, the number of molecules of fluorescein-coupled BSA-NES inside one nucleus is 0.5 × (0.3 × 10−6) × (6 × 1023) × (12.5 × 10−12) = 2.3 × 106, and the number of nuclear entry events per NPC per second is 2.3 × 106/(3,000 × 120) = ≈3.5.
Figure 5
The imported BSA-NES can be reexported. Reexport of fluorescein-conjugated BSA-NES was assayed in permeabilized cells by loading nuclei for 15 min by using the inverted-export assay as described for Fig. 3, followed by immediate fixation (A); alternatively, cells were washed, incubated for an additional 15 min at room temperature in the presence of 2 μM CRM1 and 2.5 μM RanQ69L-GTP, and then fixed (B). Nuclei were scanned with a confocal fluorescence microscope (63× oil objective).
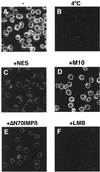
Figure 6
The import of BSA-NES is sensitive to inhibitors of NES-mediated protein export. Fluorescein-labeled BSA-NES was mixed on ice with the recombinant factors CRM1 and RanQ69L-GTP and added to digitonin-permeabilized cells. In the reaction shown in C, a 200-fold molar excess of NES peptide was added. In D, the same concentration of the mutant M10 peptide was used instead. (E) Import cells were preincubated for 5 min with 4 μM of importin β71–876 and washed before addition of the reaction mix. In F, leptomycin B (LMB) was added to a final concentration of 10 μM. This concentration of LMB does not affect nuclear import of BSA-NLS in Xenopus extracts (data not shown). Nuclear transport assays were allowed to proceed for 20 min at either 22°C (A and C–F) or on ice (B), and cells were fixed immediately with paraformaldehyde. Final concentrations of factors were 2.5 μM RanQ69L-GTP, 2 μM CRM1, and 250 nM BSA-NES. Nuclei were scanned with a confocal fluorescence microscope (63× oil objective).
Figure 7
A model for nucleocytoplasmic transport. In this model, the translocation through the pore involves multiple, reversible interactions between the receptor–cargo complex and proteins of the NPC. No directionality is built into these reactions, but the vectoriality is ensured by a Ran-regulated irreversible last step. Although no consumption of high-energy phosphates is needed for translocation through the pore, the energy potential for import comes from the high nuclear Ran-GTP concentration, whereas export is coupled directly to the RanGAP/RanBP1 induced GTP hydrolysis in the cytoplasm. By the addition of cytoplasmic Ran-GTP, the Ran gradient is inverted, and nuclear translocation of export substrates can occur. Such a model is akin to transport mechanisms of ions through membranes where electrochemical gradients are used to pump ions against their concentration gradient (for details, see Results and Discussion).
Similar articles
- Receptor-mediated substrate translocation through the nuclear pore complex without nucleotide triphosphate hydrolysis.
Englmeier L, Olivo JC, Mattaj IW. Englmeier L, et al. Curr Biol. 1999 Jan 14;9(1):30-41. doi: 10.1016/s0960-9822(99)80044-x. Curr Biol. 1999. PMID: 9889120 - Generation of GTP-Ran for nuclear protein import.
Moore MS. Moore MS. Science. 1996 Apr 5;272(5258):47. doi: 10.1126/science.272.5258.47. Science. 1996. PMID: 8600534 No abstract available. - NTF2 mediates nuclear import of Ran.
Ribbeck K, Lipowsky G, Kent HM, Stewart M, Görlich D. Ribbeck K, et al. EMBO J. 1998 Nov 16;17(22):6587-98. doi: 10.1093/emboj/17.22.6587. EMBO J. 1998. PMID: 9822603 Free PMC article. - Transport of macromolecules between the nucleus and the cytoplasm.
Izaurralde E, Adam S. Izaurralde E, et al. RNA. 1998 Apr;4(4):351-64. RNA. 1998. PMID: 9630243 Free PMC article. Review. - Nuclear import and export pathways.
Moroianu J. Moroianu J. J Cell Biochem. 1999;Suppl 32-33:76-83. doi: 10.1002/(sici)1097-4644(1999)75:32+<76::aid-jcb10>3.3.co;2-h. J Cell Biochem. 1999. PMID: 10629106 Review.
Cited by
- The yeast nuclear pore complex and transport through it.
Aitchison JD, Rout MP. Aitchison JD, et al. Genetics. 2012 Mar;190(3):855-83. doi: 10.1534/genetics.111.127803. Genetics. 2012. PMID: 22419078 Free PMC article. - Identification of a small molecule inhibitor of importin β mediated nuclear import by confocal on-bead screening of tagged one-bead one-compound libraries.
Hintersteiner M, Ambrus G, Bednenko J, Schmied M, Knox AJ, Meisner NC, Gstach H, Seifert JM, Singer EL, Gerace L, Auer M. Hintersteiner M, et al. ACS Chem Biol. 2010 Oct 15;5(10):967-79. doi: 10.1021/cb100094k. ACS Chem Biol. 2010. PMID: 20677820 Free PMC article. - Biochemical characterization of the Ran-RanBP1-RanGAP system: are RanBP proteins and the acidic tail of RanGAP required for the Ran-RanGAP GTPase reaction?
Seewald MJ, Kraemer A, Farkasovsky M, Körner C, Wittinghofer A, Vetter IR. Seewald MJ, et al. Mol Cell Biol. 2003 Nov;23(22):8124-36. doi: 10.1128/MCB.23.22.8124-8136.2003. Mol Cell Biol. 2003. PMID: 14585972 Free PMC article. - The strategy for coupling the RanGTP gradient to nuclear protein export.
Becskei A, Mattaj IW. Becskei A, et al. Proc Natl Acad Sci U S A. 2003 Feb 18;100(4):1717-22. doi: 10.1073/pnas.252766999. Epub 2003 Jan 31. Proc Natl Acad Sci U S A. 2003. PMID: 12563037 Free PMC article. - In vivo imaging of labelled endogenous β-actin mRNA during nucleocytoplasmic transport.
Grünwald D, Singer RH. Grünwald D, et al. Nature. 2010 Sep 30;467(7315):604-7. doi: 10.1038/nature09438. Epub 2010 Sep 15. Nature. 2010. PMID: 20844488 Free PMC article.
References
- Panté N, Aebi U. Crit Rev Biochem Mol Biol. 1996;31:153–199. - PubMed
- Nigg E A. Nature (London) 1997;386:779–787. - PubMed
- Mattaj I W, Englmeier L. Annu Rev Biochem. 1998;67:265–306. - PubMed
- Weis K. Trends Biochem Sci. 1998;23:185–189. - PubMed
- Bischoff F R, Ponstingl H. Nature (London) 1991;354:80–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous