c-fos-induced growth factor/vascular endothelial growth factor D induces angiogenesis in vivo and in vitro - PubMed (original) (raw)
c-fos-induced growth factor/vascular endothelial growth factor D induces angiogenesis in vivo and in vitro
L Marconcini et al. Proc Natl Acad Sci U S A. 1999.
Abstract
c-fos-induced growth factor/vascular endothelial growth factor D (Figf/Vegf-D) is a secreted factor of the VEGF family that binds to the vessel and lymphatic receptors VEGFR-2 and VEGFR-3. Here we report that Figf/Vegf-D is a potent angiogenic factor in rabbit cornea in vivo in a dose-dependent manner. In vitro Figf/Vegf-D induces tyrosine phosphorylation of VEGFR-2 and VEGFR-3 in primary human umbilical cord vein endothelial cells (HUVECs) and in an immortal cell line derived from Kaposi's sarcoma lesion (KS-IMM). The treatment of HUVECs with Figf/Vegf-D induces dose-dependent cell growth. Figf/VEGF-D also induces HUVEC elongation and branching to form an extensive network of capillary-like cords in three-dimensional matrix. In KS-IMM cells Figf/Vegf-D treatment results in dose-dependent mitogenic and motogenic activities. Taken together with the previous observations that Figf/Vegf-D expression is under the control of the nuclear oncogene c-fos, our data uncover a link between a nuclear oncogene and angiogenesis, suggesting that Figf/Vegf-D may play a critical role in tumor cell growth and invasion.
Figures
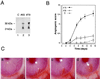
Figure 1
Implanted Figf/Vegf-D-expressing cells induce neovascularization in rabbit corneas. (A) Figf/Vegf-D expressed in CHO cells. Equal volumes of culture supernatants from clones 65 and 79 were precipitated and analyzed by Western blot using an anti-Figf/Vegf-D rabbit polyclonal antiserum. (B) CHO cells (4 × 104) expressing Figf/Vegf-D were surgically implanted into the corneas. New blood vessel growth was recorded every other day with a slit lamp stereomicroscope. Angiogenic scores were calculated on the basis of the number of vessels and their growth rate and plotted versus time (for experimental details see Materials and Methods). Angiogenic score data are the mean values obtained from the response scored in all animals in this study. C, CHO mock transfectant clone; #65, clone expressing low levels of Figf/Vegf-D (0.1 ng/ml protein in supernatant); #79 clone expressing higher levels of Figf/Vegf-D (approximately 0.5 ng/ml protein in supernatant). (C) Pictures of rabbit corneas from a representative experiment. (a) Corneal implant of CHO mock transfectant. Clone 79 promotes and sustains vascular growth over time at day 6 (b), 9 (c), and 14 (d). Corneas were photographed with a stereomicroscope. Magnification: ×18.
Figure 2
Figf/Vegf-D sustains dose-dependent angiogenesis in vivo. (A) Supernatant of S. cerevisiae yeast strains expressing Figf/Vegf-D and Figf/Vegf-D mutant as indicated. (B) The angiogenic activity of various concentrations of Figf/Vegf-D were tested as slow-release preparations in the rabbit cornea assay. (C) Angiogenic activity of 200 and 400 ng/pellet of Figf/Vegf-D N160P. (D) Angiogenic activity of 200 ng/pellet of VEGF-A121 and VEGF-A165 is shown for comparison. Angiogenic score data are the mean values obtained from the responses scored in all animals in this study. Variations were below 10% of the mean values. Angiogenic scores are calculated as described in Fig. 1 and in Materials and Methods.
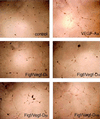
Figure 3
Figf/Vegf-D-induced endothelial cell morphological changes. VEGF-A or Figf/Vegf-D were added to HUVECs cultured in three-dimensional Matrigel in low serum conditions. Photographs were taken 24 h after Figf/Vegf-D treatment. Protein concentrations (ng/ml) used are indicated.
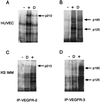
Figure 4
Figf/Vegf-D-induced tyrosine phosphorylation of VEGFR-2 and VEGFR-3. HUVECs and KS-IMM cells were incubated with Figf/Vegf-D. After stimulation receptors were immunoprecipitated with antireceptor antibodies and analyzed by Western blotting with an antiphosphotyrosine mAb. (A and B) Phosphorylation of VEGFR-2 and VEGFR-3 in HUVECs. (C and D) Phosphorylation of VEGFR-2 and VEGFR-3 in KS-IMM cells. Positive control (+) and Figf/Vegf-D stimulation (D) is indicated. Arrows denote the position of the phosphorylated 210-kDa VEGFR-2 protein and the positions of the phosphorylated, proteolytically processed 125-kDa and unprocessed 195-kDa forms of VEGFR-3.
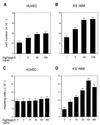
Figure 5
Figf/Vegf-D-induced cell proliferation and chemotactic activity. (A and B) Proliferative effects of Figf/Vegf-D were assayed on HUVECs and KS-IMM cells as indicated. Experiments were performed in medium containing 1% FCS. After 72 h cells were enumerated by using a Coulter counter and values represent the mean (±SEM) of triplicate samples. (C and D) Cells were seeded in the upper wells of a 48-well micro chemotaxis Boyden chamber and incubated for 7 h at 37°C in medium containing 1% FCS. The lower wells contained the indicated concentrations of Figf/VEGF-D. Cells migrating through a polycarbonate membrane with a pore size of 5 μm were quantified by staining the cells with Giemsa solution and counting was performed on a light microscope of five high-power fields (×100). The results are expressed as the mean ± 1 SD of three independent experiments performed in triplicate.
Similar articles
- Vascular endothelial growth factor-C stimulates the migration and proliferation of Kaposi's sarcoma cells.
Marchiò S, Primo L, Pagano M, Palestro G, Albini A, Veikkola T, Cascone I, Alitalo K, Bussolino F. Marchiò S, et al. J Biol Chem. 1999 Sep 24;274(39):27617-22. doi: 10.1074/jbc.274.39.27617. J Biol Chem. 1999. PMID: 10488101 - Vascular endothelial growth factor C induces angiogenesis in vivo.
Cao Y, Linden P, Farnebo J, Cao R, Eriksson A, Kumar V, Qi JH, Claesson-Welsh L, Alitalo K. Cao Y, et al. Proc Natl Acad Sci U S A. 1998 Nov 24;95(24):14389-94. doi: 10.1073/pnas.95.24.14389. Proc Natl Acad Sci U S A. 1998. PMID: 9826710 Free PMC article. - Identification of a peptide blocking vascular endothelial growth factor (VEGF)-mediated angiogenesis.
Binétruy-Tournaire R, Demangel C, Malavaud B, Vassy R, Rouyre S, Kraemer M, Plouët J, Derbin C, Perret G, Mazié JC. Binétruy-Tournaire R, et al. EMBO J. 2000 Apr 3;19(7):1525-33. doi: 10.1093/emboj/19.7.1525. EMBO J. 2000. PMID: 10747021 Free PMC article. - Possible involvement of VEGF-FLT tyrosine kinase receptor system in normal and tumor angiogenesis.
Shibuya M, Seetharam L, Ishii Y, Sawano A, Gotoh N, Matsushime H, Yamaguchi S. Shibuya M, et al. Princess Takamatsu Symp. 1994;24:162-70. Princess Takamatsu Symp. 1994. PMID: 8983073 Review. - Vascular Endothelial Growth Factor-D (VEGF-D): An Angiogenesis Bypass in Malignant Tumors.
Bokhari SMZ, Hamar P. Bokhari SMZ, et al. Int J Mol Sci. 2023 Aug 28;24(17):13317. doi: 10.3390/ijms241713317. Int J Mol Sci. 2023. PMID: 37686121 Free PMC article. Review.
Cited by
- Trichosanthes kirilowii Extract Promotes Wound Healing through the Phosphorylation of ERK1/2 in Keratinocytes.
Kim M, Kim JG, Kim KY. Kim M, et al. Biomimetics (Basel). 2022 Oct 7;7(4):154. doi: 10.3390/biomimetics7040154. Biomimetics (Basel). 2022. PMID: 36278711 Free PMC article. - Kaposin-B enhances the PROX1 mRNA stability during lymphatic reprogramming of vascular endothelial cells by Kaposi's sarcoma herpes virus.
Yoo J, Kang J, Lee HN, Aguilar B, Kafka D, Lee S, Choi I, Lee J, Ramu S, Haas J, Koh CJ, Hong YK. Yoo J, et al. PLoS Pathog. 2010 Aug 12;6(8):e1001046. doi: 10.1371/journal.ppat.1001046. PLoS Pathog. 2010. PMID: 20730087 Free PMC article. - The lymphangiogenic vascular endothelial growth factors VEGF-C and -D are ligands for the integrin alpha9beta1.
Vlahakis NE, Young BA, Atakilit A, Sheppard D. Vlahakis NE, et al. J Biol Chem. 2005 Feb 11;280(6):4544-52. doi: 10.1074/jbc.M412816200. Epub 2004 Dec 6. J Biol Chem. 2005. PMID: 15590642 Free PMC article. - The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease.
Holmes DI, Zachary I. Holmes DI, et al. Genome Biol. 2005;6(2):209. doi: 10.1186/gb-2005-6-2-209. Epub 2005 Feb 1. Genome Biol. 2005. PMID: 15693956 Free PMC article. Review. - Interleukin-1beta-mediated inhibition of the processes of angiogenesis in cardiac microvascular endothelial cells.
Mountain DJ, Singh M, Singh K. Mountain DJ, et al. Life Sci. 2008 Jun 20;82(25-26):1224-30. doi: 10.1016/j.lfs.2008.04.008. Epub 2008 Apr 23. Life Sci. 2008. PMID: 18501931 Free PMC article.
References
- Ferrara N, Davis-Smyth T. Endocr Rev. 1997;18:4–25. - PubMed
- Bussolino F, Mantovani A, Persico G. Trends Biochem Sci. 1997;22:251–256. - PubMed
- Risau W. Nature (London) 1997;386:671–674. - PubMed
- Folkman J. EXS. 1997;79:1–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous