Cytomegalovirus encodes a potent alpha chemokine - PubMed (original) (raw)
Cytomegalovirus encodes a potent alpha chemokine
M E Penfold et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Cytomegalovirus is a widespread opportunistic pathogen affecting immunocompromised individuals in whom neutrophils may mediate virus dissemination and contribute to progression of disease. Recent sequence analysis suggests that genes absent or altered in attenuated strains may influence pathogenesis. We have found two genes, UL146 and UL147, whose products have sequence similarity to alpha (CXC) chemokines. UL146 encodes a protein, designated vCXC-1, that is a 117-aa glycoprotein secreted into the culture medium as a late gene product, where its presence correlates with the ability to attract human neutrophils. Recombinant vCXC-1 is a fully functional chemokine, inducing calcium mobilization, chemotaxis, and degranulation of neutrophils. High-affinity vCXC-1 binding is shown to be mediated via CXCR2, but not CXCR1. vCXC-1 exhibits a potency approaching that of human IL-8. As the first example of a virus-encoded alpha chemokine, vCXC-1 may ensure the active recruitment of neutrophils during cytomegalovirus infection, thereby providing for efficient dissemination during acute infection and accounting for the prominence of this leukocyte subset in cytomegalovirus disease.
Figures
Figure 1
Schematic representation of strain Toledo and Toledo-derived mutant virus genomes. (A, upper line) CMV strain Toledo genome structure with the invertable L and S genome segments indicated by arrows and inverted repeats (ab-b′a′c′-ca) indicated by boxes. Expanded region is the ULb′ region, with ORFs depicted as arrows. (B) Structures of the recombinant viruses. Nucleotide numbers correspond to Toledo sequences (accession no. U33331). (C) Sequence comparison between UL146 (Toledo), UL146 (Towne), UL147, and human IL-8. Identities are boxed, and the two most likely signal peptide cleavage sites predicted by computer algorithm are marked (Y18 and T23). The cleavage site of mature IL-8 and FLAG insertion site in UL146 (Toledo) also are indicated.
Figure 2
Expression of FLAG-tagged vCXC-1 by Tol146FLAG. (A) Detection of FLAG-tagged vCXC-1 in cell lysates by immunoblot with anti-FLAG M2 Ab. Whole-cell lysates were harvested from uninfected (Mock) or Tol146FLAG-infected cells at 24, 48, 72, and 96 hpi. Cell lysates also were collected at 96 hpi from Tol146 FLAG-infected cells cultured in the presence of 660 μM phosphonoformic acid (96 PFA). (B) Structural analysis of FLAG-tagged vCXC-1. Infected cell lysates and virions, enriched by sucrose gradient, were harvested at 96 hpi and treated with PNGase F as indicated (+). The proteins were immunoblotted with anti-FLAG M2 Ab [Coomassie staining confirmed the composition of the virion preparation: viral (pp65) but not cellular (β-actin) antigens were detected by immunoblotting.] (C) Immunoprecipitation of FLAG-tagged vCXC-1 from medium (Secreted) and cell lysates. Protein was harvested at 96 hpi from mock-infected, Toledo, and Tol146FLAG-infected fibroblasts, treated with PNGase F as indicated (+), and precipitated with anti-FLAG M2 Ab. The arrow labeled “Ig” marks a nonspecific reaction with Ig light chain. The arrow below denotes the position of 25,000- to 27,000-MW secreted form of FLAG-tagged vCXC-1. (D) Mock- and Tol146FLAG-infected fibroblasts were stained with anti-FLAG M2 Ab and goat anti-mouse FITC at 96 hpi. Toledo-infected and antibody controls were negative.
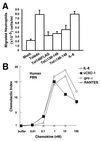
Figure 3
vCXC-1-induced chemotaxis of human PBN. (A) Chemotaxis of neutrophils toward cell-free supernatants. IL-8 (12 nM) was added to mock-infected cell supernatants as a positive control (IL-8). (Bars = 2 SD.) (B) Induction of migration of PBN by recombinant vCXC-1(T23 form) compared with IL-8 and gro α. RANTES, a CC chemokine, is included as negative control. Chemotactic index represents the fold increase in migration over a basal level in the absence of chemokine.
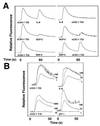
Figure 4
Analysis of active form of recombinant vCXC-1 and its effects on human PBN. (A) Heterologous desensitization profiles of listed chemokines on PBN. (Left) T23 form of vCXC-1 added as initial agonist, followed 60 sec later by other human chemokines, as listed. (Right) Human chemokines added first, followed by the T23 form of vCXC-1. (B) Cytoplasmic calcium induction by Y18 and T23 forms of vCXC-1; comparison with human IL-8 and gro α [chemokine (in nM) is indicated on the right of each data set].
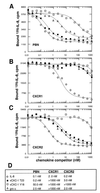
Figure 5
Equilibrium-binding competition profiles of vCXC-1 on human PBN and individual CXC chemokine receptor-bearing cells. Unlabeled chemokines were incubated along with 0.1 nM 125I-IL-8 by using primary human neutrophils (A) or transfected murine NSO cells stably expressing human chemokine receptors CXCR1 (B) or CXCR2 (C). (D) Competition constants (_K_i) for each interaction as derived from Scatchard analysis of the data.
Similar articles
- Sequence variability of human cytomegalovirus UL146 and UL147 genes in low-passage clinical isolates.
He R, Ruan Q, Qi Y, Ma YP, Huang YJ, Sun ZR, Ji YH. He R, et al. Intervirology. 2006;49(4):215-23. doi: 10.1159/000091468. Epub 2006 Feb 16. Intervirology. 2006. PMID: 16491016 - Novel Human Cytomegalovirus Viral Chemokines, vCXCL-1s, Display Functional Selectivity for Neutrophil Signaling and Function.
Heo J, Dogra P, Masi TJ, Pitt EA, de Kruijf P, Smit MJ, Sparer TE. Heo J, et al. J Immunol. 2015 Jul 1;195(1):227-36. doi: 10.4049/jimmunol.1400291. Epub 2015 May 18. J Immunol. 2015. PMID: 25987741 Free PMC article. - Fatal attraction: cytomegalovirus-encoded chemokine homologs.
Saederup N, Mocarski ES Jr. Saederup N, et al. Curr Top Microbiol Immunol. 2002;269:235-56. doi: 10.1007/978-3-642-59421-2_14. Curr Top Microbiol Immunol. 2002. PMID: 12224512 - The human cytomegalovirus chemokine receptor homolog encoded by US27.
Stegman JR, Margulies BJ. Stegman JR, et al. Virus Genes. 2017 Aug;53(4):516-521. doi: 10.1007/s11262-017-1462-y. Epub 2017 Apr 26. Virus Genes. 2017. PMID: 28447191 Review. - Neutrophil receptors for interleukin-8 and related CXC chemokines.
Murphy PM. Murphy PM. Semin Hematol. 1997 Oct;34(4):311-8. Semin Hematol. 1997. PMID: 9347581 Review.
Cited by
- CXCL12 signaling in the development of the nervous system.
Mithal DS, Banisadr G, Miller RJ. Mithal DS, et al. J Neuroimmune Pharmacol. 2012 Dec;7(4):820-34. doi: 10.1007/s11481-011-9336-x. Epub 2012 Jan 21. J Neuroimmune Pharmacol. 2012. PMID: 22270883 Free PMC article. Review. - Cytomegalovirus CC chemokine promotes immune cell migration.
Vomaske J, Denton M, Kreklywich C, Andoh T, Osborn JM, Chen D, Messaoudi I, Orloff SL, Streblow DN. Vomaske J, et al. J Virol. 2012 Nov;86(21):11833-44. doi: 10.1128/JVI.00452-12. Epub 2012 Aug 22. J Virol. 2012. PMID: 22915808 Free PMC article. - Human cytomegalovirus IE86 attenuates virus- and tumor necrosis factor alpha-induced NFkappaB-dependent gene expression.
Taylor RT, Bresnahan WA. Taylor RT, et al. J Virol. 2006 Nov;80(21):10763-71. doi: 10.1128/JVI.01195-06. J Virol. 2006. PMID: 17041226 Free PMC article. - The role of cytomegalovirus in angiogenesis.
Caposio P, Orloff SL, Streblow DN. Caposio P, et al. Virus Res. 2011 May;157(2):204-11. doi: 10.1016/j.virusres.2010.09.011. Epub 2010 Oct 1. Virus Res. 2011. PMID: 20869406 Free PMC article. Review. - Potent immunosuppressive activities of cytomegalovirus-encoded interleukin-10.
Spencer JV, Lockridge KM, Barry PA, Lin G, Tsang M, Penfold ME, Schall TJ. Spencer JV, et al. J Virol. 2002 Feb;76(3):1285-92. doi: 10.1128/jvi.76.3.1285-1292.2002. J Virol. 2002. PMID: 11773404 Free PMC article.
References
- Taylor-Wiedeman J, Sissons J G, Borysiewicz L K, Sinclair J H. J Gen Virol. 1991;72:2059–2064. - PubMed
- Dankner W M, McCutchan J A, Richman D D, Hirata K, Spector S A. J Infect Dis. 1990;161:31–36. - PubMed
- Theodossiou C, Temeck B, Vargas H, Yang J, Vargas M, Hahn S, Pass H. Am J Gastroenterol. 1995;90:1174–1176. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials