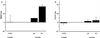Inhibition of nitric-oxide synthase 2 by aminoguanidine provides neuroprotection of retinal ganglion cells in a rat model of chronic glaucoma - PubMed (original) (raw)
Inhibition of nitric-oxide synthase 2 by aminoguanidine provides neuroprotection of retinal ganglion cells in a rat model of chronic glaucoma
A H Neufeld et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Glaucoma is an optic neuropathy with cupping of the optic disk, degeneration of retinal ganglion cells, and characteristic visual field loss. Because elevated intraocular pressure (IOP) is a major risk factor for progression of glaucoma, treatment has been based on lowering IOP. We previously demonstrated inducible nitric-oxide synthase (NOS-2) in the optic nerve heads from human glaucomatous eyes and from rat eyes with chronic, moderately elevated IOP. Using this rat model of unilateral glaucoma, we treated a group of animals for 6 months with aminoguanidine, a relatively specific inhibitor of NOS-2, and compared them with an untreated group. At 6 months, untreated animals had pallor and cupping of the optic disks in the eyes with elevated IOP. Eyes of aminoguanidine-treated animals with similar elevations of IOP appeared normal. We quantitated retinal ganglion cell loss by retrograde labeling with Fluoro-Gold. When compared with their contralateral control eyes with normal IOP, eyes with elevated IOP in the untreated group lost 36% of their retinal ganglion cells; the eyes with similarly elevated IOP in the aminoguanidine-treated group lost less than 10% of their retinal ganglion cells. Pharmacological neuroprotection by inhibition of NOS-2 may prove useful for the treatment of patients with glaucoma.
Figures
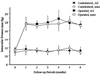
Figure 1
Chronic, moderately elevated IOP in rat eyes that had three episcleral vessels cauterized, unilaterally (Operated), vs. the opposite eye (Contralateral). IOP was measured on anesthetized animals with a Mentor Classic 30 Pneumotonometer. In one group (n = 8), aminoguanidine (AG) was added to the drinking water; in the control group (n = 8), nothing was added to the drinking water (none). Throughout the 6-month follow-up, IOP was elevated in all Operated eyes compared with Contralateral eyes (P < 0.01). Elevated IOP was not different in the group treated with aminoguanidine vs. the group not treated pharmacologically (_P_ > 0.1). All values are mean ± SD.
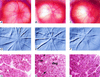
Figure 2
Fundus photographs and histological cross-sections of optic nerves from eyes with normal IOP and chronic, moderately elevated IOP, with and without treating the animals with aminoguanidine for 6 months. Color fundus photographs (A–C) and red-free photographs for relief imaging (D–F) were taken on anesthetized animals. The highlights in B and C are reflections of light from the camera. Optic nerve cross-sections (G–I) were stained for myelin profiles (magnification, ×1,250). The peripheral edge of the optic nerve is to the right side of each photomicrograph, opposite the label (G–I). Arrows in H point to the approximate boundary between normal-appearing optic nerve tissue and degenerated (deg) tissue. Pictures from the same eyes are as follows: A, D, and G are from an eye with normal IOP and contralateral to B, E, and H, which are from an eye with chronic, moderately elevated IOP for 6 months from an animal not pharmacologically treated. C, F, and I are from an eye with chronic, moderately elevated IOP for 6 months from an animal treated with aminoguanidine.
Figure 3
Percentage of retinal ganglion cells (RGC) lost at 6 months in rat eyes with chronic, moderately elevated IOP. One week after Fluoro-Gold labeling, retinal ganglion cells were counted in both eyes of each animal (n = 6 for the aminoguanidine-treated group; n = 6 for the untreated group). RGCs were counted in the peripheral retina (A), approximately 4.0 mm from the optic disk, and in the central retina (B), approximately 1.0 mm from the optic disk. To demonstrate uniform bilateral distribution of Fluoro-Gold in retinal ganglion cells after bilateral injection into the superior colliculus, a separate group of normal animals (n = 6) was studied. These animals had normal IOP bilaterally. The mean ± SD of the ratios of retinal ganglion cell counts for left and right eyes were 0.99 ± 0.16 in the peripheral retina and 1.02 ± 0.05 in the central retina. These values were used for the determination of percent RGC lost at “0 Month” (CONT); % RGC lost = 1 − [RGC density in eye with elevated IOP/RGC density in eye with normal IOP] × 100%. In peripheral retina at 6 months, percent RGC lost in eyes with elevated IOP from animals that were not treated pharmacologically (NT) is statistically significantly different than control (P < 0.01), whereas percent RGC lost in eyes with elevated IOP from animals that were treated with aminoguanidine (AG) is not statistically significantly different than control (_P_ > 0.1). All values are mean ± SD.
Comment in
- Nitric-oxide synthase and neurodegeneration/neuroprotection.
Kaufman PL. Kaufman PL. Proc Natl Acad Sci U S A. 1999 Aug 17;96(17):9455-6. doi: 10.1073/pnas.96.17.9455. Proc Natl Acad Sci U S A. 1999. PMID: 10449712 Free PMC article. Review. No abstract available.
Similar articles
- Isoforms of nitric oxide synthase in the optic nerves of rat eyes with chronic moderately elevated intraocular pressure.
Shareef S, Sawada A, Neufeld AH. Shareef S, et al. Invest Ophthalmol Vis Sci. 1999 Nov;40(12):2884-91. Invest Ophthalmol Vis Sci. 1999. PMID: 10549648 - Loss of retinal ganglion cells following retinal ischemia: the role of inducible nitric oxide synthase.
Neufeld AH, Kawai Si, Das S, Vora S, Gachie E, Connor JR, Manning PT. Neufeld AH, et al. Exp Eye Res. 2002 Nov;75(5):521-8. doi: 10.1006/exer.2002.2042. Exp Eye Res. 2002. PMID: 12457864 - A prodrug of a selective inhibitor of inducible nitric oxide synthase is neuroprotective in the rat model of glaucoma.
Neufeld AH, Das S, Vora S, Gachie E, Kawai S, Manning PT, Connor JR. Neufeld AH, et al. J Glaucoma. 2002 Jun;11(3):221-5. doi: 10.1097/00061198-200206000-00010. J Glaucoma. 2002. PMID: 12140399 - Pharmacologic neuroprotection with an inhibitor of nitric oxide synthase for the treatment of glaucoma.
Neufeld AH. Neufeld AH. Brain Res Bull. 2004 Feb 15;62(6):455-9. doi: 10.1016/j.brainresbull.2003.07.005. Brain Res Bull. 2004. PMID: 15036557 Review. - Nitric oxide: a potential mediator of retinal ganglion cell damage in glaucoma.
Neufeld AH. Neufeld AH. Surv Ophthalmol. 1999 Jun;43 Suppl 1:S129-35. doi: 10.1016/s0039-6257(99)00010-7. Surv Ophthalmol. 1999. PMID: 10416755 Review.
Cited by
- Methylene Blue Reduces Electroretinogram Distortion and Ganglion Cell Death in a Rat Model of Glaucoma.
Nakamura R, Ciranna NS, Fernández JC, Peláez R, Pérez-Sala Á, Bobadilla M, López-Costa JJ, Loidl CF, Martínez A, Rey-Funes M. Nakamura R, et al. Biomedicines. 2024 Sep 2;12(9):1983. doi: 10.3390/biomedicines12091983. Biomedicines. 2024. PMID: 39335498 Free PMC article. - Novel therapeutic targets for primary open-angle glaucoma identified through multicenter proteome-wide mendelian randomization.
Yuan W, Li J, Gao S, Sun W, Zhao F. Yuan W, et al. Front Pharmacol. 2024 Aug 16;15:1428472. doi: 10.3389/fphar.2024.1428472. eCollection 2024. Front Pharmacol. 2024. PMID: 39221148 Free PMC article. - The PKG Inhibitor CN238 Affords Functional Protection of Photoreceptors and Ganglion Cells against Retinal Degeneration.
Tolone A, Haq W, Fachinger A, Roy A, Kesh S, Rentsch A, Wucherpfennig S, Zhu Y, Groten J, Schwede F, Tomar T, Herberg FW, Nache V, Paquet-Durand F. Tolone A, et al. Int J Mol Sci. 2023 Oct 17;24(20):15277. doi: 10.3390/ijms242015277. Int J Mol Sci. 2023. PMID: 37894958 Free PMC article. - Nitric oxide: an old drug but with new horizons in ophthalmology-a narrative review.
Lee S, Park CY. Lee S, et al. Ann Transl Med. 2023 Aug 30;11(10):352. doi: 10.21037/atm-22-5634. Epub 2023 Jun 6. Ann Transl Med. 2023. PMID: 37675299 Free PMC article. Review. - Exploring the Relationship between Anti-VEGF Therapy and Glaucoma: Implications for Management Strategies.
Daka Q, Špegel N, Atanasovska Velkovska M, Steblovnik T, Kolko M, Neziri B, Cvenkel B. Daka Q, et al. J Clin Med. 2023 Jul 14;12(14):4674. doi: 10.3390/jcm12144674. J Clin Med. 2023. PMID: 37510790 Free PMC article. Review.
References
- Armaly M F, Krueger D E, Maunder L, Becker B, Hetherington J, Jr, Kolker A E, Levene R Z, Maumenee A E, Pollack I P, Shaffer R N. Arch Ophthalmol. 1980;98:2163–2171. - PubMed
- Quigley H A, Addicks E M, Green W R, Maumenee A E. Arch Ophthalmol. 1981;99:635–649. - PubMed
- Anderson D R. Am J Ophthalmol. 1989;108:485–495. - PubMed
- Alward W L. N Engl J Med. 1998;339:1298–1307. - PubMed
- Adachi K, Fujita Y, Morizane C, Akaike A, Ueda M, Satoh M, Masai H, Kashii S, Honda Y. Eur J Pharmacol. 1998;350:53–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical