Suppression of Ras-induced apoptosis by the Rac GTPase - PubMed (original) (raw)
Suppression of Ras-induced apoptosis by the Rac GTPase
T Joneson et al. Mol Cell Biol. 1999 Sep.
Abstract
Ras is an essential component of signal transduction pathways that control cell proliferation, differentiation, and survival. In this study we have examined the cellular responses to high-intensity Ras signaling. Expression of increasing amounts of the oncogenic form of human HRas, HRasV12, results in a dose-dependent induction of apoptosis in both primary and immortalized cells. The induction of apoptosis by HRasV12 is blocked by activated Rac and potentiated by dominant interfering Rac. The ability of Rac to suppress Ras-induced apoptosis is dependent on effector pathway(s) controlled by the insert region and is linked to the activation of NF-kappaB. The apoptotic effect of HRasV12 requires the activation of both the ERK and JNK mitogen-activated protein kinase cascade and is independent of p53. These results demonstrate a role for Rac in controlling signals that are necessary for cell survival, and suggest a mechanism by which Rac activity can confer growth advantage to cells transformed by the ras oncogene.
Figures
FIG. 1
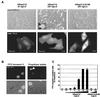
High-intensity Ras signaling induces apoptosis. (A) Serum-starved REF-52 cells were microinjected with the indicated expression plasmids. (Top panels) Phase-contrast micrographs of the injected areas visualized 24 h after injection. Apoptotic cells appear as highly refractile, loosely adherent spheres. The inset shows a high-magnification image of an HRasV12-expressing REF-52 cell undergoing apoptosis. Note the convolution of the cellular surface and the presence of membrane-bound apoptotic bodies (arrowheads). (Bottom panels) Immunofluorescence micrographs of REF-52 cells injected with the indicated expression plasmids. Cells were fixed and stained 5 h after injection. Differences in the levels of protein expression are indicated by the differences in the intensity of the fluorescence signal. The nuclear exclusion staining pattern in cells expressing HRasV12R186 indicates cytosolic localization. Numbers at lower right denote exposure time. (B) Apoptotic responses induced by HRasV12. Serum-starved REF-52 cells were microinjected with HRasV12 expression plasmid (20 ng/μl) and stained with FITC-annexin V or propidium iodide 24 h after injection. For each stain, the fluorescence micrographs and the corresponding phase-contrast micrographs are shown in the lower panels. The propidium iodide staining shows a condensed apoptotic nucleus (arrowhead). (C) Dose-response relationship between expression levels of HRasV12 and induction of apoptosis. Serum-starved REF-52 cells were microinjected with the indicated concentrations of expression plasmids. Twenty-four hours after injection, cells were incubated with FITC-annexin V. The percentage of annexin V-positive cells was determined by counting FITC-annexin V-positive cells as a proportion of the total number of cells in the injected area. The results are the means of three independent experiments in which at least 100 cells were scored for each condition. Error bars represent standard deviations. Typically, 60% of the cells in the injected areas expressed the exogenous protein as determined by immunofluorescence staining.
FIG. 2
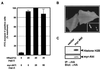
Akt is not sufficient to protect against HRasV12-induced apoptosis. (A) REF-52 cells were injected with HRasV12 (20 ng/μl) alone or with increasing concentration of myr-Akt (25 and 50 ng/μl). Twenty-four hours after injection, cells were stained with annexin V and the number of apoptotic cells was determined as described in the legend to Fig. 1C. (B) Immunofluorescence micrographs of REF-52 cells injected with HA-tagged myr-Akt or HA-tagged wild-type Akt (inset) demonstrating plasma membrane and cytosolic localization, respectively. Cells were fixed 12 h after injection and stained with anti-HA antibodies. (C) myr-Akt is constitutively active. HEK-293 cells were transfected with HA epitope-tagged myr-Akt, and kinase activity was determined by immune complex kinase assay using histone 2B as a substrate. IP, immunoprecipitation; αHA, anti-HA antibody.
FIG. 3
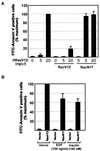
Rac is necessary and sufficient for suppression of apoptosis. (A) Effect of constitutively active (RacV12) and dominant interfering (RacN17) Rac on Ras-induced apoptosis. Expression vectors encoding the indicated Rac mutants (25 ng/μl) were microinjected together with the indicated concentrations of HRasV12 expression plasmid. (B) Role of Rac in growth factor-mediated cell survival. Serum-starved REF-52 cells were injected with plasmid mixtures containing expression vectors for the growth factor receptor (50 ng/μl) or HRasV12 (20 ng/μl) with or without Rac N17 (50 ng/μl). Six hours after injection, cells were stimulated with EGF (100 ng/ml) or insulin (100 nM). Twenty-four hours after injection, cells were stained with annexin V and the number of apoptotic cells was determined as described in the legend to Fig. 1C. Results are the means of three independent experiments in which at least 100 cells were scored for each condition. Percent maximum corresponds to 47% ± 3.7% (A) 56% ± 9.8% (B) of the cells in the injected area. Error bars represent standard deviations.
FIG. 4
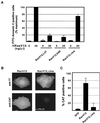
The insert region of Rac is required for suppression of Ras-induced apoptosis and NF-κB activation. (A) Role of Rac effector pathways in the protection against Ras-induced apoptosis. Serum-starved REF-52 cells were microinjected with a mixture of plasmids containing a high (20 ng/μl) or low (5 ng/μl) concentration of HRasV12 in combination with or the indicated Rac mutants (25 ng/μl). FITC-annexin V-positive cells were scored 24 h after injection. Values correspond to the means of three independent experiments, and the error bars represent the standard deviations. At least 100 cells were scored per condition in each experiment. Percent maximum corresponds to 39% ± 4.9% of the cells in the injected area. (B) Serum-starved REF-52 cells were microinjected with a mixture of expression plasmids containing an NF-κB–CAT reporter construct (50 ng/μl) and the indicated T7 epitope-tagged Rac mutants or with CMV-GFP as a negative control (50 ng/μl). The injected cells were fixed 16 h postinjection and costained with a mixture of mouse antibodies to T7 epitope-tagged Rac mutants and rabbit antibodies to CAT followed by fluorescein-conjugated antibodies to mouse IgG and rhodamine-conjugated antibodies to rabbit IgG. (C) Quantitation of NF-κB dependent transcription. Cells expressing the proteins as determined by immunofluorescence or autofluorescence in the case of GFP were scored for CAT costaining. Results shown are the means of three independent experiments in which at least 50 cells were scored, and the error bars represent the standard deviations.
FIG. 5
Effects of NF-κB activation and Rho family GTPases on Ras-induced apoptosis. (A) REF-52 cells were injected with the indicated concentrations of HRasV12 in combination with the NF-κB subunit (c-Rel) (50 ng/μl) or a dominant interfering mutant of the IκB kinase kinase (DI-NIK) (50 ng/μl). (B) REF-52 cells were coinjected with the indicated constructs and with the indicated constitutively activated or dominant interfering mutant of Rac, RhoA, or CDC42 (50 ng/μl). Twenty-four hours after injection, annexin V-positive cells were counted. Results are the means of three independent experiments in which at least 100 cells were scored for each condition. Percent maximum corresponds to 42% ± 3.7% of the cells in the injected area (A and B). Error bars represent standard deviations.
FIG. 6
ERK and JNK MAP kinase cascades are both required for Ras-induced apoptosis. (A) REF-52 cells were microinjected with an expression plasmid for HRasV12, HRasV12,S35, or HRasV12,C40 (20 ng/μl), Raf-CAAX (50 ng/μl), or MKK7 (50 ng/μl). Each Ras mutant was also coinjected with phosphatases specific for MAP kinase family members: MKP-1, MKP-3, or a kinase-defective mutant of JNK1 (50 ng/μl). Twenty-four hours after injection, cells were stained with annexin V and the number of apoptotic cells was determined as described in the legend to Fig. 1C. Results are the means of three independent experiments in which at least 100 cells were scored for each condition. Error bars represent standard deviations. (B) Effects of MKP-1 and MKP-3 on Ras-induced JNK and ERK kinase activity. COS-1 cells were transfected with 1 μg of expression vector for HRasV12 and either 10 μg of FLAG epitope-tagged JNK1 or 1 μg HA epitope-tagged ERK2 with either 1 or 5 μg of MKP-1 or MKP-3. Kinase activity was determined by immune complex kinase assay using MBP and c-Jun as substrates. (C) MKK7 is a potent activator of JNK. COS-1 cells were transfected with 10 μg of FLAG-tagged JNK1 and 10 μg of RacV12 or 5 μg of MKK7.
Similar articles
- Role of small GTP binding proteins in the growth-promoting and antiapoptotic actions of gastrin.
Stepan V, Ramamoorthy S, Pausawasdi N, Logsdon CD, Askari FK, Todisco A. Stepan V, et al. Am J Physiol Gastrointest Liver Physiol. 2004 Sep;287(3):G715-25. doi: 10.1152/ajpgi.00169.2003. Am J Physiol Gastrointest Liver Physiol. 2004. PMID: 15331357 - Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade.
Lamarche N, Tapon N, Stowers L, Burbelo PD, Aspenström P, Bridges T, Chant J, Hall A. Lamarche N, et al. Cell. 1996 Nov 1;87(3):519-29. doi: 10.1016/s0092-8674(00)81371-9. Cell. 1996. PMID: 8898204 - The small GTPases Cdc42Hs, Rac1 and RhoG delineate Raf-independent pathways that cooperate to transform NIH3T3 cells.
Roux P, Gauthier-Rouvière C, Doucet-Brutin S, Fort P. Roux P, et al. Curr Biol. 1997 Sep 1;7(9):629-37. doi: 10.1016/s0960-9822(06)00289-2. Curr Biol. 1997. PMID: 9285711 - Regulation of phosphorylation pathways by p21 GTPases. The p21 Ras-related Rho subfamily and its role in phosphorylation signalling pathways.
Lim L, Manser E, Leung T, Hall C. Lim L, et al. Eur J Biochem. 1996 Dec 1;242(2):171-85. doi: 10.1111/j.1432-1033.1996.0171r.x. Eur J Biochem. 1996. PMID: 8973630 Review. - Rho small G protein and cytoskeletal control.
Takai Y, Kaibuchi K, Sasaki T, Tanaka K, Shirataki H, Nakanishi H. Takai Y, et al. Princess Takamatsu Symp. 1994;24:338-50. Princess Takamatsu Symp. 1994. PMID: 8983086 Review.
Cited by
- Rac1 promotes the survival of H9c2 cells during serum deficiency targeting JNK/c-JUN/Cyclin-D1 and AKT2/MCL1 pathways.
Zhao J, Jie Q, Li G, Li Y, Liu B, Li H, Luo J, Qin X, Li Z, Wei Y. Zhao J, et al. Int J Med Sci. 2018 Jun 23;15(10):1062-1071. doi: 10.7150/ijms.25527. eCollection 2018. Int J Med Sci. 2018. PMID: 30013448 Free PMC article. - Hyperactivation of ERK by multiple mechanisms is toxic to RTK-RAS mutation-driven lung adenocarcinoma cells.
Unni AM, Harbourne B, Oh MH, Wild S, Ferrarone JR, Lockwood WW, Varmus H. Unni AM, et al. Elife. 2018 Nov 26;7:e33718. doi: 10.7554/eLife.33718. Elife. 2018. PMID: 30475204 Free PMC article. - The role of FilGAP-filamin A interactions in mechanoprotection.
Shifrin Y, Arora PD, Ohta Y, Calderwood DA, McCulloch CA. Shifrin Y, et al. Mol Biol Cell. 2009 Mar;20(5):1269-79. doi: 10.1091/mbc.e08-08-0872. Epub 2009 Jan 14. Mol Biol Cell. 2009. PMID: 19144823 Free PMC article. - NF-kappaB and cancer: how intimate is this relationship.
Prasad S, Ravindran J, Aggarwal BB. Prasad S, et al. Mol Cell Biochem. 2010 Mar;336(1-2):25-37. doi: 10.1007/s11010-009-0267-2. Epub 2009 Oct 8. Mol Cell Biochem. 2010. PMID: 19823771 Free PMC article. Review. - Elevated phospholipase D activity in H-Ras- but not K-Ras-transformed cells by the synergistic action of RalA and ARF6.
Xu L, Frankel P, Jackson D, Rotunda T, Boshans RL, D'Souza-Schorey C, Foster DA. Xu L, et al. Mol Cell Biol. 2003 Jan;23(2):645-54. doi: 10.1128/MCB.23.2.645-654.2003. Mol Cell Biol. 2003. PMID: 12509462 Free PMC article.
References
- Baichwal V R, Baeuerle P A. Apoptosis: activate NF-κB or die? Curr Biol. 1997;7:R94–R96. - PubMed
- Baldwin A S. The NF-kappaB and I kappaB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. - PubMed
- Campbell S L, Khosravi-Far R, Rossman K L, Clark G J, Der C J. Increasing complexity of Ras signaling. Oncogene. 1998;17:1395–1413. - PubMed
- Chen C Y, Faller D V. Direction of p21ras-generated signals towards cell growth or apoptosis is determined by protein kinase C and Bcl-2. Oncogene. 1995;11:1487–1498. - PubMed
- Derijard M, Hibi, Wu I, Barrett T, Su B, Deng T, Karin M, Davis R. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-jun activation domain. Cell. 1994;76:1025–1037. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous