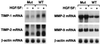The von Hippel-Lindau tumor suppressor gene inhibits hepatocyte growth factor/scatter factor-induced invasion and branching morphogenesis in renal carcinoma cells - PubMed (original) (raw)
. 1999 Sep;19(9):5902-12.
doi: 10.1128/MCB.19.9.5902.
M Jeffers, P H Wang, C Gong, G A Taylor, L M Roessler, R Stearman, J R Vasselli, W G Stetler-Stevenson, W G Kaelin Jr, W M Linehan, R D Klausner, J R Gnarra, G F Vande Woude
Affiliations
- PMID: 10454537
- PMCID: PMC84441
- DOI: 10.1128/MCB.19.9.5902
The von Hippel-Lindau tumor suppressor gene inhibits hepatocyte growth factor/scatter factor-induced invasion and branching morphogenesis in renal carcinoma cells
S Koochekpour et al. Mol Cell Biol. 1999 Sep.
Abstract
Loss of function in the von Hippel-Lindau (VHL) tumor suppressor gene occurs in familial and most sporadic renal cell carcinomas (RCCs). VHL has been linked to the regulation of cell cycle cessation (G(0)) and to control of expression of various mRNAs such as for vascular endothelial growth factor. RCC cells express the Met receptor tyrosine kinase, and Met mediates invasion and branching morphogenesis in many cell types in response to hepatocyte growth factor/scatter factor (HGF/SF). We examined the HGF/SF responsiveness of RCC cells containing endogenous mutated (mut) forms of the VHL protein (VHL-negative RCC) with that of isogenic cells expressing exogenous wild-type (wt) VHL (VHL-positive RCC). We found that VHL-negative 786-0 and UOK-101 RCC cells were highly invasive through growth factor-reduced (GFR) Matrigel-coated filters and exhibited an extensive branching morphogenesis phenotype in response to HGF/SF in the three-dimensional (3D) GFR Matrigel cultures. In contrast, the phenotypes of A498 VHL-negative RCC cells were weaker, and isogenic RCC cells ectopically expressing wt VHL did not respond at all. We found that all VHL-negative RCC cells expressed reduced levels of tissue inhibitor of metalloproteinase 2 (TIMP-2) relative to the wt VHL-positive cells, implicating VHL in the regulation of this molecule. However, consistent with the more invasive phenotype of the 786-0 and UOK-101 VHL-negative RCC cells, the levels of TIMP-1 and TIMP-2 were reduced and levels of the matrix metalloproteinases 2 and 9 were elevated compared to the noninvasive VHL-positive RCC cells. Moreover, recombinant TIMPs completely blocked HGF/SF-mediated branching morphogenesis, while neutralizing antibodies to the TIMPs stimulated HGF/SF-mediated invasion in vitro. Thus, the loss of the VHL tumor suppressor gene is central to changes that control tissue invasiveness, and a more invasive phenotype requires additional genetic changes seen in some but not all RCC lines. These studies also demonstrate a synergy between the loss of VHL function and Met signaling.
Figures
FIG. 1
VHL regulation of HGF/SF-mediated RCC cell branching morphogenesis. 786-0 RCC cells were mixed in a GFR Matrigel solution and transferred to tissue culture plates. After a 30-min incubation at 37°C, the cells were fed with DMEM–10% FBS alone (Control) or supplemented with 40 ng of HGF/SF per well. After 3 days, the representative fields were photographed. Magnification, ×348. Each experiment was performed in triplicate, and the assays were repeated three times. WT, VHL-positive 786-0 RCC cells (WT-7); Mut, VHL-negative 786-0 RCC cells (ARZ-2).
FIG. 2
VHL regulation of HGF/SF-mediated RCC cell invasion in vitro. After overnight serum starvation, 104 786-0 cells were placed on top of the transwell filters coated with GFR Matrigel (20 μg) in a final volume of 100 μl of DMEM–0.1% BSA alone (Control) or supplemented with HGF/SF (20 ng/ml). The lower compartment of each transwell unit contained 500 μl of DMEM. After a 20-h incubation, the noninvading cells on the upper surface of the filter were removed and the invasive cells attached to the lower surface of the filter were stained. A representative field was photographed. Magnification, ×172. Each sample was assayed in triplicate, and the assays were repeated three times. Mut, VHL-negative 786-0 RCC cells (ARZ-2); WT, VHL-positive 786-0 RCC cells (WT-7).
FIG. 3
Met expression and signaling in 786-0 RCC cells. (A) 786-0 RCC cells, after overnight serum deprivation, were washed and fed with fresh DMEM–0.1% BSA alone or supplemented with 200 ng of HGF/SF per ml for 10 min, and 0.5 mg of cell lysate was immunoprecipitated with anti-Met C-28 and subjected to SDS-polyacrylamide gel electrophoresis and immunoblotting with anti-P-Tyr (top). The membrane was then stripped and reprobed with anti-C-28 antibody (bottom). Mut, VHL-negative RCC cells; WT, VHL-positive 786-0 RCC cells. (B) Fos induction in VHL-negative or -positive 786-0 RCC. Subconfluent cultures of cells were serum starved overnight and incubated for 30 min with DMEM alone or supplemented with either 200 ng of HGF/SF per ml or 20% FBS. Northern analysis was performed as detailed in Materials and Methods. A 1-kb _Pst_I fragment of pFos-1 was used as the probe, and an equal amount of loading per lane was demonstrated by reprobing the membrane with glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Mut, VHL-negative 786-0 RCC cells (ARZ-2); WT, VHL-positive 786-0 RCC cells (WT-7).
FIG. 4
uPA and uPAR induction in 786-0 RCC cells. Subconfluent cultures of cells were incubated for 7 h at 37°C in DMEM–10% FBS supplemented with 200 ng of HGF/SF per ml or unsupplemented. Total RNA extraction and Northern hybridization were performed as described in Materials and Methods. The blots were probed or reprobed with a 32P-labeled human uPA probe, a human uPAR probe, and a human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) probe (52). Mut, VHL-negative 786-0 RCC cells; WT, VHL-positive 786-0 RCC cells.
FIG. 5
TIMP expression in RCC cells and the influence of HGF/SF. Western analysis of TIMP-2 (A) and TIMP-1 (B) proteins in culture supernatants was performed. Subconfluent cultures in DMEM–0.1% BSA were incubated in the presence or absence of 20 ng of HGF per ml for 12, 24, 36, or 48 h. Only the 36-h sample is presented. Conditioned medium was concentrated and 25 μg of protein was resolved under reducing conditions on an SDS–12% polyacrylamide gel. Western analysis was performed as described in Materials and Methods. Anti-TIMP-2 (A) and TIMP-1 (B) at 1 μg/ml were used as primary antibodies. Mut, VHL-negative RCC; WT, VHL-positive RCC. pRC, ARZ-2, and ARZ-3 are VHL-negative 786-0 RCC cells. P (parental) and Fs are VHL-negative UOK-101 RCC cells; WT is an VHL-positive RCC cell line. IDV relative integrated density value. Comparative densitometric analyses were performed with an Alpha Imager 2000 version 3.2. All values were normalized to 786-0 WT-7.
FIG. 6
MMP activity in VHL-positive and VHL-negative RCC. (A) Conditioned medium from HT1080 fibrosarcoma cells was used as a positive control (49). Gelatin zymography of serum-free conditioned medium collected from cells incubated at 37°C for 12, 24, 36, or 48 h in DMEM supplemented with 0.2% (wt/vol) lactalbumin hydrolysate with or without HGF/SF (20 ng/ml) is shown. Only the 36-h sample is shown. Culture supernatants were processed, and 25 μg was subjected to gelatin zymography under nonreducing and nondenaturing conditions. Prestained marker proteins showed approximate molecular masses as indicated. Proteolytic activity, the light bands against the stained background, correspond to MMP-2 and MMP-9. The MMP-2 bands appear as doublets, representing the heavier, inactive form and the fully activated molecule. Linearity of the enzyme-substrate reaction was demonstrated by serial dilution of APMA-activated supernatant. At any dilution, the proteolytic band in the VHL-negative cells was higher than in the VHL-positive cells. In parallel experiments we used either 10 mM 1,10-phenathroline or EDTA to inhibit MMP activity. This completely blocked gelatinase activity (data not shown). The HT1080 fibrosarcoma cell line was used as a positive control for collagenase expression, as was purified human MMP-2 and MMP-9. Comparative densitometric analyses were performed with an Alpha Imager 2000; all values were normalized to VHL-negative ARZ-3 cells. pRC, ARZ-2, and ARZ-3 are VHL-negative 786-0 RCC cell lines; P (parental) and Fs are VHL-negative UOK-101 RCC cell lines; WT is a VHL-positive RCC cell line. (B) Western analysis of MMP-2 and MMP-9 in culture supernatant was performed as in Figure 5 captions. A representative immunoblot at 36 h is shown here. Cell lines are as in panel A.
FIG. 7
Expression of TIMP and MMP mRNA in VHL-positive and VHL-negative RCC cells. Subconfluent cultures of 786-0 RCC cells were incubated for 12, 24, 36, or 48 h in serum-free medium supplemented with HGF/SF (20 ng/ml) or unsupplemented. Only results for the 36-h samples are presented. Total RNA extraction and Northern hybridization were performed as described in Materials and Methods. The blots were probed or reprobed with a 32P-labeled human TIMP-1, TIMP-2, MMP-2, MMP-9, and β-actin probe. Mut, VHL-negative 786-0 RCC cells (ARZ-2); WT, VHL-positive 786-0 RCC cells (WT-7).
FIG. 8
TIMP-1 and TIMP-2 neutralizing antibodies and 786-0 RCC cell in vitro invasiveness. Invasion assays were performed in collagen type IV by the method described in the legend to Fig. 2. Heat-inactivated neutralizing rabbit anti-human TIMP-1 and TIMP-2 antibodies (or two different preimmune rabbit sera) were used at 5% (vol/vol) dilutions in the upper compartment. A greater number of invading cells per filter in the VHL-negative cells than in the VHL-positive cells in the presence of HGF/SF (20 ng/ml) and neutralizing TIMP-1 (Neut-T1) alone or in combination with neutralizing TIMP-2 (Neut-T2) was found (see Table 1). WT, VHL-positive 786-0 RCC cells (WT-7); Mut, VHL-negative 786-0 RCC cells (ARZ-2).
FIG. 9
TIMP-1 and TIMP-2 and branching morphogenesis in VHL-positive and VHL-negative RCC cells. Branching morphogenesis assays on 786-0 RCC cells were performed as described in the legend to Fig. 1. TIMP-1 (6.4 μg/ml) or TIMP-2 (10 μg/ml) were included in both the gel and the supernatant. Control wells received equal volumes of PBS. The assay was performed for all wt and mut VHL cell lines (data shown for only VHL-negative 786-0 RCC cells). For any cell line, treatment of cells expressing wt VHL with TIMP-1 or TIMP-2 resulted in no response (data not shown). Each experiment was performed in quadruplicate, and the assays were repeated three times.
Similar articles
- Inactivation of the von Hippel-Lindau (VHL) tumour suppressor gene and allelic losses at chromosome arm 3p in primary renal cell carcinoma: evidence for a VHL-independent pathway in clear cell renal tumourigenesis.
Clifford SC, Prowse AH, Affara NA, Buys CH, Maher ER. Clifford SC, et al. Genes Chromosomes Cancer. 1998 Jul;22(3):200-9. doi: 10.1002/(sici)1098-2264(199807)22:3<200::aid-gcc5>3.0.co;2-#. Genes Chromosomes Cancer. 1998. PMID: 9624531 - Identification of cyclin D1 and other novel targets for the von Hippel-Lindau tumor suppressor gene by expression array analysis and investigation of cyclin D1 genotype as a modifier in von Hippel-Lindau disease.
Zatyka M, da Silva NF, Clifford SC, Morris MR, Wiesener MS, Eckardt KU, Houlston RS, Richards FM, Latif F, Maher ER. Zatyka M, et al. Cancer Res. 2002 Jul 1;62(13):3803-11. Cancer Res. 2002. PMID: 12097293 - The von Hippel-Lindau tumor suppressor gene product represses oncogenic beta-catenin signaling in renal carcinoma cells.
Peruzzi B, Athauda G, Bottaro DP. Peruzzi B, et al. Proc Natl Acad Sci U S A. 2006 Sep 26;103(39):14531-6. doi: 10.1073/pnas.0606850103. Epub 2006 Sep 18. Proc Natl Acad Sci U S A. 2006. PMID: 16983094 Free PMC article. - The von Hippel-Lindau tumor suppressor gene and kidney cancer.
Kaelin WG Jr. Kaelin WG Jr. Clin Cancer Res. 2004 Sep 15;10(18 Pt 2):6290S-5S. doi: 10.1158/1078-0432.CCR-sup-040025. Clin Cancer Res. 2004. PMID: 15448019 Review. - Von Hippel-Lindau disease and sporadic renal cell carcinoma.
Zbar B. Zbar B. Cancer Surv. 1995;25:219-32. Cancer Surv. 1995. PMID: 8718521 Review.
Cited by
- Clinical relevance of hepsin and hepatocyte growth factor activator inhibitor type 2 expression in renal cell carcinoma.
Betsunoh H, Mukai S, Akiyama Y, Fukushima T, Minamiguchi N, Hasui Y, Osada Y, Kataoka H. Betsunoh H, et al. Cancer Sci. 2007 Apr;98(4):491-8. doi: 10.1111/j.1349-7006.2007.00412.x. Epub 2007 Feb 16. Cancer Sci. 2007. PMID: 17309599 Free PMC article. - Cabozantinib in the treatment of advanced renal cell carcinoma: design, development, and potential place in the therapy.
Grassi P, Verzoni E, Ratta R, Mennitto A, de Braud F, Procopio G. Grassi P, et al. Drug Des Devel Ther. 2016 Jul 5;10:2167-72. doi: 10.2147/DDDT.S104225. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 27462141 Free PMC article. Review. - VHL promotes E2 box-dependent E-cadherin transcription by HIF-mediated regulation of SIP1 and snail.
Evans AJ, Russell RC, Roche O, Burry TN, Fish JE, Chow VW, Kim WY, Saravanan A, Maynard MA, Gervais ML, Sufan RI, Roberts AM, Wilson LA, Betten M, Vandewalle C, Berx G, Marsden PA, Irwin MS, Teh BT, Jewett MA, Ohh M. Evans AJ, et al. Mol Cell Biol. 2007 Jan;27(1):157-69. doi: 10.1128/MCB.00892-06. Epub 2006 Oct 23. Mol Cell Biol. 2007. PMID: 17060462 Free PMC article. - Inadequate activation of the GTPase RhoA contributes to the lack of fibronectin matrix assembly in von Hippel-Lindau protein-defective renal cancer cells.
Feijóo-Cuaresma M, Méndez F, Maqueda A, Esteban MA, Naranjo-Suarez S, Castellanos MC, del Cerro MH, Vazquez SN, García-Pardo A, Landázuri MO, Calzada MJ. Feijóo-Cuaresma M, et al. J Biol Chem. 2008 Sep 5;283(36):24982-90. doi: 10.1074/jbc.M709390200. Epub 2008 Jun 19. J Biol Chem. 2008. PMID: 18567581 Free PMC article. - Renal involvement in tuberous sclerosis complex and von Hippel-Lindau disease: shared disease mechanisms?
Siroky BJ, Czyzyk-Krzeska MF, Bissler JJ. Siroky BJ, et al. Nat Clin Pract Nephrol. 2009 Mar;5(3):143-56. doi: 10.1038/ncpneph1032. Nat Clin Pract Nephrol. 2009. PMID: 19240728 Review.
References
- Aznavoorian S, Murphy A N, Stetler-Stevenson W G, Liotta L A. Molecular aspects of tumor cell invasion and metastasis. Cancer. 1993;71:1368–1383. - PubMed
- Droz D, Patey N, Paraf F, Chretien Y, Gogusev J. Composition of extracellular matrix and distribution of cell adhesion molecules in renal cell tumors. Lab Investig. 1994;71:710–718. - PubMed
- Friedhelm B, Riethmacher S, Isenmann S, Aaguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into limb bud. Nature. 1995;376:768–771. - PubMed
- Gnarra J R, Duan D, Weng Y, Humphrey J S, Chen D Y, Lee S, Pause A, Dudley C F, Latif F, Kuzmin I, Schmidt L, Duh F M, Stackhouse T, Chen F, Kishida T, Wei M H, Lerman M I, Zbar B, Klausner R D, Linehan W M. Molecular cloning of the von Hippel-Lindau tumor suppressor gene and its role in renal carcinoma. Biochim Biophys Acta. 1996;1242:201–210. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous