Lactoferrin-lipid A-lipopolysaccharide interaction: inhibition by anti-human lactoferrin monoclonal antibody AGM 10.14 - PubMed (original) (raw)
Lactoferrin-lipid A-lipopolysaccharide interaction: inhibition by anti-human lactoferrin monoclonal antibody AGM 10.14
D Caccavo et al. Infect Immun. 1999 Sep.
Abstract
Lactoferrin (LF) is a glycoprotein that exerts both bacteriostatic and bactericidal activities. The interaction of LF with lipopolysaccharide (LPS) of gram-negative bacteria seems to play a crucial role in the bactericidal effect. In this study, we evaluated, by means of an enzyme-linked immunosorbent assay, the binding of biotinylated LF to the S (smooth) and R (rough) (Ra, Rb, Rc, Rd1, Rd2, and Re) forms of LPS and different lipid A preparations. In addition, the effects of two monoclonal antibodies (AGM 10.14, an immunoglobulin G1 [IgG1] antibody, and AGM 2.29, an IgG2b antibody), directed against spatially distant epitopes of human LF, on the LF-lipid A or LF-LPS interaction were evaluated. The results showed that biotinylated LF specifically binds to solid-phase lipid A, as this interaction was prevented in a dose-dependent fashion by either soluble uncoupled LF or lipid A. The binding of LF to S-form LPS was markedly weaker than that to lipid A. Moreover, the rate of LF binding to R-form LPS was inversely related to core length. The results suggest that the polysaccharide O chain as well as oligosaccharide core structures may interfere with the LF-lipid A interaction. In addition, we found that soluble lipid A also inhibited LF binding to immobilized LPS, demonstrating that, in the whole LPS structure, the lipid A region contains the major determinant recognized by LF. AGM 10.14 inhibited LF binding to lipid A and LPS in a dose-dependent fashion, indicating that this monoclonal antibody recognizes an epitope involved in the binding of LF to lipid A or some epitope in its close vicinity. In contrast, AGM 2.29, even in a molar excess, did not prevent the binding of LF to lipid A or LPS. Therefore, AGM 10.14 may represent a useful tool for neutralizing selectively the binding of LF to lipid A. In addition, the use of such a monoclonal antibody could allow better elucidation of the consequences of the LF-lipid A interaction.
Figures
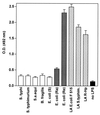
FIG. 1
Binding of bhLF to different forms of LPS or lipid A (LA). Each bar is representative of the mean OD ± standard deviation for three separate experiments. bhLF was used at a concentration of 300 ng/ml (100 μl/well). Nonspecific binding was assessed by adding bhLF to wells from which the coating antigen was omitted (black bar). S.a. equi, S. abortus-equi; S. typhim., S. typhimurium; R-Hp, H. pylori R-form.
FIG. 2
(A) Cross-blocking of bhLF binding to solid-phase lipid A by uncoupled hLF. E. coli F 515 lipid A-coated wells were preincubated for 1 h with 100 μl of increasing concentrations (twofold increments) of uncoupled hLF (solid circles) or BSA (negative control; open circles) per well. Without removal of the competitor, bhLF (30 ng/well) was added, and binding was evaluated as reported in Materials and Methods. Nonspecific binding was subtracted. Data are representative results obtained in duplicate in three independent experiments. (B) Inhibition of bhLF binding to solid-phase lipid A by soluble lipid A. A constant amount of bhLF (final concentration, 300 ng/ml) was mixed with increasing concentrations (fourfold increments) of soluble E. coli F 515 lipid A (solid circles) or BSA (negative control; open circles) and incubated for 1 h. Then, 100 μl of this mixture was added to E. coli F 515 lipid A-coated wells, and binding was evaluated as reported in Materials and Methods. Nonspecific binding was subtracted. Data are representative results obtained in duplicate in three independent experiments.
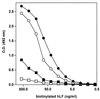
FIG. 3
Comparative dose-dependent binding of bhLF to lipid A, Re LPS, and Ra LPS. One hundred microliters of bhLF (twofold dilutions) was added to wells coated with E. coli F 515 lipid A (solid circles), E. coli F 515 Re LPS (open circles), or E. coli EH 100 Ra LPS (solid squares). Binding was evaluated as reported in Materials and Methods. Nonspecific binding was assessed by adding bhLF to wells from which the coating antigen was omitted (open squares).
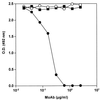
FIG. 4
Inhibition of bhLF binding to solid-phase lipid A by anti-hLF MAbs (MoAb). A constant amount of bhLF (final concentration, 300 ng/ml) was mixed with increasing concentrations (twofold increments) of anti-hLF MAb AGM 10.14 (solid circles), anti-hLF MAb AGM 2.29 (open circles), or control MAb 16 D7 (solid squares) and incubated for 1 h. Then, 100 μl of this mixture was added to E. coli F 515 lipid A-coated wells, and binding was evaluated as reported in Materials and Methods. Nonspecific binding was subtracted. Data are representative results obtained in duplicate in three independent experiments.
Similar articles
- Two spatially distant epitopes of human lactoferrin.
Caccavo D, Afeltra A, Guido F, Di Monaco C, Ferri GM, Amoroso A, Vaccaro F, Bonomo L. Caccavo D, et al. Hybridoma. 1996 Aug;15(4):263-9. doi: 10.1089/hyb.1996.15.263. Hybridoma. 1996. PMID: 8880213 - Lactoferrin is a lipid A-binding protein.
Appelmelk BJ, An YQ, Geerts M, Thijs BG, de Boer HA, MacLaren DM, de Graaff J, Nuijens JH. Appelmelk BJ, et al. Infect Immun. 1994 Jun;62(6):2628-32. doi: 10.1128/iai.62.6.2628-2632.1994. Infect Immun. 1994. PMID: 8188389 Free PMC article. - Biophysical characterization of lipopolysaccharide and lipid A inactivation by lactoferrin.
Brandenburg K, Jürgens G, Müller M, Fukuoka S, Koch MH. Brandenburg K, et al. Biol Chem. 2001 Aug;382(8):1215-25. doi: 10.1515/BC.2001.152. Biol Chem. 2001. PMID: 11592403 - Antimicrobial and immunoregulatory functions of lactoferrin and its potential therapeutic application.
Caccavo D, Pellegrino NM, Altamura M, Rigon A, Amati L, Amoroso A, Jirillo E. Caccavo D, et al. J Endotoxin Res. 2002;8(6):403-17. doi: 10.1179/096805102125001000. J Endotoxin Res. 2002. PMID: 12542852 Review. - Immunoregulatory role of lactoferrin-lipopolysaccharide interactions.
Puddu P, Latorre D, Valenti P, Gessani S. Puddu P, et al. Biometals. 2010 Jun;23(3):387-97. doi: 10.1007/s10534-010-9307-3. Epub 2010 Feb 27. Biometals. 2010. PMID: 20191308 Review.
Cited by
- Resolvin E1-induced intestinal alkaline phosphatase promotes resolution of inflammation through LPS detoxification.
Campbell EL, MacManus CF, Kominsky DJ, Keely S, Glover LE, Bowers BE, Scully M, Bruyninckx WJ, Colgan SP. Campbell EL, et al. Proc Natl Acad Sci U S A. 2010 Aug 10;107(32):14298-303. doi: 10.1073/pnas.0914730107. Epub 2010 Jul 26. Proc Natl Acad Sci U S A. 2010. PMID: 20660763 Free PMC article. - Crosstalk between the Resident Microbiota and the Immune Cells Regulates Female Genital Tract Health.
Santacroce L, Palmirotta R, Bottalico L, Charitos IA, Colella M, Topi S, Jirillo E. Santacroce L, et al. Life (Basel). 2023 Jul 9;13(7):1531. doi: 10.3390/life13071531. Life (Basel). 2023. PMID: 37511906 Free PMC article. Review. - Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock.
Van Amersfoort ES, Van Berkel TJ, Kuiper J. Van Amersfoort ES, et al. Clin Microbiol Rev. 2003 Jul;16(3):379-414. doi: 10.1128/CMR.16.3.379-414.2003. Clin Microbiol Rev. 2003. PMID: 12857774 Free PMC article. Review. - High Serum Lipopolysaccharide-Binding Protein Level in Chronic Hepatitis C Viral Infection Is Reduced by Anti-Viral Treatments.
Nien HC, Hsu SJ, Su TH, Yang PJ, Sheu JC, Wang JT, Chow LP, Chen CL, Kao JH, Yang WS. Nien HC, et al. PLoS One. 2017 Jan 20;12(1):e0170028. doi: 10.1371/journal.pone.0170028. eCollection 2017. PLoS One. 2017. PMID: 28107471 Free PMC article. - N-linked glycosylation is required for c1 inhibitor-mediated protection from endotoxin shock in mice.
Liu D, Gu X, Scafidi J, Davis AE 3rd. Liu D, et al. Infect Immun. 2004 Apr;72(4):1946-55. doi: 10.1128/IAI.72.4.1946-1955.2004. Infect Immun. 2004. PMID: 15039314 Free PMC article.
References
- Arnold R R, Cole M F, McGhee J R. A bactericidal role for human lactoferrin. Science. 1977;197:263–265. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources