Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae - PubMed (original) (raw)
Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae
A H Tu et al. Infect Immun. 1999 Sep.
Abstract
Pneumococcal surface protein A (PspA) is a surface-exposed protein virulence factor for Streptococcus pneumoniae. In this study, no significant depletion of serum complement was observed for the serum of mice infected with pneumococci that express PspA. In contrast, in mice infected with an isogenic strain of pneumococci lacking PspA, significant activation of serum complement was detected within 30 min after infection. Also, the PspA-deficient strain but not the PspA-expressing strain was cleared from the blood within 6 h. The contribution of PspA to pneumococcal virulence was further investigated by using mice deficient for C5, C3, or factor B. In mice deficient for C3 or factor B, PspA-negative pneumococci became fully virulent. In contrast, in C5-deficient mice as in wild-type mice, PspA-deficient pneumococci were avirulent. These in vivo data suggest that, in nonimmune mice infected with pneumococci, PspA interferes with complement-dependent host defense mechanisms mediated by factor B. Immunoblots of pneumococci opsonized in vitro suggested that more C3b was deposited on PspA-negative than on PspA-positive pneumococci. This was observed with and without anticapsular antibody. Furthermore, processing of the alpha chain of C3b was reduced in the presence of PspA. We propose that PspA exerts its virulence function by interfering with deposition of C3b onto pneumococci and/or by inhibiting formation of a fully functional alternative pathway C3 convertase. By blocking recruitment of the alternative pathway, PspA reduces the amount of C3b deposited onto pneumococci, thereby reducing the effectiveness of complement receptor-mediated pathways of clearance.
Figures
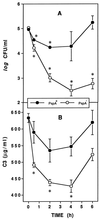
FIG. 1
PspA inhibits complement activation and clearance of pneumococci. XID mice were infected intravenously with approximately 105 CFU of PspA+ or PspA− pneumococci, and blood was collected to quantitate bacteremia (A) and serum C3 (B). The data are presented as the means ± standard errors of the means from a total of 15 mice per group (two experiments). Asterisks indicate significant decreases in bacteremia or serum C3 concentrations compared to 0-h (preinfection) values (P < 0.05, paired t test).
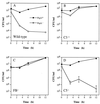
FIG. 2
PspA+ and PspA− pneumococci (closed and open circles, respectively) have equal virulence in C3− and FB− mice. Mice were infected intravenously with approximately 107 CFU of PspA+ or PspA− pneumococci and bled at various times thereafter to quantitate bacteremia. The data are presented as the means ± standard errors of the means for groups of two to five mice.
FIG. 3
Western blot analysis of C3 deposition onto PspA+ and PspA− pneumococci. Pneumococci were exposed to XID mouse serum in the absence (lanes 2 to 8) or presence (lanes 9 to 20) of anticapsular antibody (Ab). Pneumococci were collected after exposure to XID mouse serum for 2 to 30 min (see Materials and Methods), boiled in reducing buffer, electrophoresed in SDS-polyacrylamide gels, transferred to nitrocellulose, and visualized with anti-C3 antibody. The right panel shows intact α and β chains of C3 present in normal mouse serum (NMS) and absent in C3− serum.
Similar articles
- The virulence function of Streptococcus pneumoniae surface protein A involves inhibition of complement activation and impairment of complement receptor-mediated protection.
Ren B, McCrory MA, Pass C, Bullard DC, Ballantyne CM, Xu Y, Briles DE, Szalai AJ. Ren B, et al. J Immunol. 2004 Dec 15;173(12):7506-12. doi: 10.4049/jimmunol.173.12.7506. J Immunol. 2004. PMID: 15585877 - Effects of PspA and antibodies to PspA on activation and deposition of complement on the pneumococcal surface.
Ren B, Szalai AJ, Hollingshead SK, Briles DE. Ren B, et al. Infect Immun. 2004 Jan;72(1):114-22. doi: 10.1128/IAI.72.1.114-122.2004. Infect Immun. 2004. PMID: 14688088 Free PMC article. - PspA and PspC minimize immune adherence and transfer of pneumococci from erythrocytes to macrophages through their effects on complement activation.
Li J, Glover DT, Szalai AJ, Hollingshead SK, Briles DE. Li J, et al. Infect Immun. 2007 Dec;75(12):5877-85. doi: 10.1128/IAI.00839-07. Epub 2007 Oct 8. Infect Immun. 2007. PMID: 17923519 Free PMC article. - Pneumococcal surface protein A inhibits complement deposition on the pneumococcal surface by competing with the binding of C-reactive protein to cell-surface phosphocholine.
Mukerji R, Mirza S, Roche AM, Widener RW, Croney CM, Rhee DK, Weiser JN, Szalai AJ, Briles DE. Mukerji R, et al. J Immunol. 2012 Dec 1;189(11):5327-35. doi: 10.4049/jimmunol.1201967. Epub 2012 Oct 26. J Immunol. 2012. PMID: 23105137 Free PMC article. - The role of antibody and complement in the reticuloendothelial clearance of pneumococci from the bloodstream.
Brown EJ, Hosea SW, Frank MM. Brown EJ, et al. Rev Infect Dis. 1983 Sep-Oct;5 Suppl 4:S797-805. doi: 10.1093/clinids/5.supplement_4.s797. Rev Infect Dis. 1983. PMID: 6356294 Review.
Cited by
- Conjugation of polysaccharide 6B from Streptococcus pneumoniae with pneumococcal surface protein A: PspA conformation and its effect on the immune response.
Perciani CT, Barazzone GC, Goulart C, Carvalho E, Cabrera-Crespo J, Gonçalves VM, Leite LC, Tanizaki MM. Perciani CT, et al. Clin Vaccine Immunol. 2013 Jun;20(6):858-66. doi: 10.1128/CVI.00754-12. Epub 2013 Apr 3. Clin Vaccine Immunol. 2013. PMID: 23554468 Free PMC article. - Diversity of PspA: mosaic genes and evidence for past recombination in Streptococcus pneumoniae.
Hollingshead SK, Becker R, Briles DE. Hollingshead SK, et al. Infect Immun. 2000 Oct;68(10):5889-900. doi: 10.1128/IAI.68.10.5889-5900.2000. Infect Immun. 2000. PMID: 10992499 Free PMC article. - Streptococcus adherence and colonization.
Nobbs AH, Lamont RJ, Jenkinson HF. Nobbs AH, et al. Microbiol Mol Biol Rev. 2009 Sep;73(3):407-50, Table of Contents. doi: 10.1128/MMBR.00014-09. Microbiol Mol Biol Rev. 2009. PMID: 19721085 Free PMC article. Review. - Pneumococci: immunology of the innate host response.
Paterson GK, Orihuela CJ. Paterson GK, et al. Respirology. 2010 Oct;15(7):1057-63. doi: 10.1111/j.1440-1843.2010.01814.x. Epub 2010 Jul 20. Respirology. 2010. PMID: 20646240 Free PMC article. Review. - Diagnosis of Streptococcus pneumoniae infection using circulating antibody secreting cells.
Kyu S, Ramonell RP, Kuruvilla M, Kraft CS, Wang YF, Falsey AR, Walsh EE, Daiss JL, Paulos S, Rajam G, Wu H, Velusamy S, Lee FE. Kyu S, et al. PLoS One. 2021 Nov 12;16(11):e0259644. doi: 10.1371/journal.pone.0259644. eCollection 2021. PLoS One. 2021. PMID: 34767590 Free PMC article.
References
- Briles, D. E., S. K. Hollingshead, E. Swiatlo, A. Brooks-Walter, A. Szalai, A. Virolainen, L. S. McDaniel, K. A. Benton, P. C. Aerts, H. van Dijk, and M. J. Crain. Pneumococcal proteins PspA and PspC: their potential for use as vaccines. In A. Tomasz and R. Austrian (ed.), Molecular biology of pneumococci and pneumococcal diseases, in press. Mary Ann Liebert Inc., Larchmont, N.Y. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI021548/AI/NIAID NIH HHS/United States
- AI-07051/AI/NIAID NIH HHS/United States
- T32 AI007051/AI/NIAID NIH HHS/United States
- R56 AI021548/AI/NIAID NIH HHS/United States
- AI-21548/AI/NIAID NIH HHS/United States
- AI-42183/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous