VAMP-7 mediates vesicular transport from endosomes to lysosomes - PubMed (original) (raw)
VAMP-7 mediates vesicular transport from endosomes to lysosomes
R J Advani et al. J Cell Biol. 1999.
Abstract
A more complete picture of the molecules that are critical for the organization of membrane compartments is beginning to emerge through the characterization of proteins in the vesicle-associated membrane protein (also called synaptobrevin) family of membrane trafficking proteins. To better understand the mechanisms of membrane trafficking within the endocytic pathway, we generated a series of monoclonal and polyclonal antibodies against the cytoplasmic domain of vesicle-associated membrane protein 7 (VAMP-7). The antibodies recognize a 25-kD membrane-associated protein in multiple tissues and cell lines. Immunohistochemical analysis reveals colocalization with a marker of late endosomes and lysosomes, lysosome-associated membrane protein 1 (LAMP-1), but not with other membrane markers, including p115 and transferrin receptor. Treatment with nocodozole or brefeldin A does not disrupt the colocalization of VAMP-7 and LAMP-1. Immunoelectron microscopy analysis shows that VAMP-7 is most concentrated in the trans-Golgi network region of the cell as well as late endosomes and transport vesicles that do not contain the mannose-6 phosphate receptor. In streptolysin- O-permeabilized cells, antibodies against VAMP-7 inhibit the breakdown of epidermal growth factor but not the recycling of transferrin. These data are consistent with a role for VAMP-7 in the vesicular transport of proteins from the early endosome to the lysosome.
Figures
Figure 1
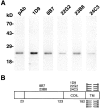
Polyclonal antibodies and mAbs specifically recognize VAMP-7. (A) 20 μg of PNS from rat kidney was probed for VAMP-7 with an affinity-purified polyclonal and five different mAbs. A 25-kD band is recognized by all antibodies. (B) The domain of VAMP-7 recognized by the mAbs was mapped. The transmembrane (TM) region corresponds to the predicted membrane anchor. The coil domain is recognized by three antibodies and a more NH2-terminal domain by two antibodies.
Figure 3
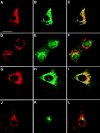
VAMP-7 partially colocalizes with an LE and lysosomal marker in NIH-3T3 cells. VAMP-7 immunoreactivity (Texas red; A, D, and G) is compared with immunofluorescence (FITC) of LAMP-1 (B), p115 (E), or TfR (H). C, F, and I are the merged images. Arrowheads in A–C point to puncta that are immunoreactive for both VAMP-7 and LAMP-1. Bar, 10 μm.
Figure 2
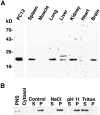
VAMP-7 is broadly expressed and associates with membranes. (A) Seven tissues and PC12 cells were probed with the polyclonal antibody for VAMP-7 expression as described in the text and Materials and Methods. 20 μg of PNS was used for each tissue. (B) PNS was fractionated into cytosolic and membrane fractions. The membrane pellet was extracted with 1.5 M NaCl, pH 11.0, or 2% Triton X-100 and centrifuged into supernatant (S) or pellet (P) fractions.
Figure 6
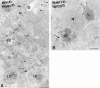
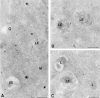
VAMP-7 colocalizes with lgp120 in LEs and is present on MPR-negative transport vesicles. PC12 cells double-immunogold–labeled for (A) the CD-MPR (MPR, 10 nm gold) and VAMP-7 (15 nm gold), and (B) VAMP-7 (10 nm gold) and lgp120 (15 nm gold). (A) The small arrowheads point to small-sized, electron-dense transport vesicles that contain the MPR but not VAMP-7. VAMP-7 is found on larger vesicles (large arrowheads) with a less electron-dense content (see also Fig. 5 C). (B) VAMP-7 colocalizes with lgp120 to the limiting membrane of an LE. The arrow points to a connection between a VAMP-7–positive vesicle that is in continuity with the vacuolar region of the LE. G, Golgi complex; L, lysosome; M, mitochondrion. Bars, 200 nm.
Figure 4
Distribution of VAMP-7 immunoreactivity in nocodozole- and BFA-treated NIH-3T3 cells. (A–F) Nocodozole-treated cells stained for VAMP-7 (A and D), LAMP-1 (B), or TfR (E). (G–L) BFA-treated cells stained for VAMP-7 (G and J), LAMP-1 (H), or TfR (K). Bar, 10 μm.
Figure 5
VAMP-7 is localized to the TGN, LEs, and transport vesicles. Ultrathin cryosections of PC12 cells immunogold-labeled (10 nm gold) for VAMP-7. In A and B, the signal for VAMP-7 was enhanced by using an intermediate swine anti–rabbit IgG antibody. (A) Overview of the perinuclear area. VAMP-7 is present in the Golgi complex (indicated by G) and many of the tubulovesicular membranes of the TGN. The open star indicates an immature secretory granule, as judged by the electron-dense cytosolic clathrin coat. The filled stars indicate mature secretory granules. No significant label is found over these secretory compartments. (B) VAMP-7 is present on the limiting membrane of an LE. (C) LE-associated vesicles. L, lysosome; M, mitochondrion. Bars, 200 nm.
Figure 7
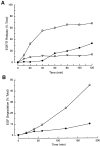
HeLa cells recycle Tf and break down EGF. (A) 125I-EGF or -Tf was loaded into cells and then chased into the supernatant. Tf was recovered in a TCA-precipitable form with a half-life of ∼20 min (open circles). EGF is recovered in two forms, TCA precipitated or intact (open triangles) and non-TCA precipitated or degraded (filled circles). The intact form of EGF rapidly appears in the cell media while the degraded form begins to appear after ∼40 min and continues to increase throughout the 120-min period. (B) Cells loaded with 125I-EGF were permeabilized with SLO. 125I-EGF degradation was measured in the presence (open circles) and absence (filled circles) of cytosol. EGF degradation proceeds with similar kinetics in permeabilized and intact cells.
Figure 8
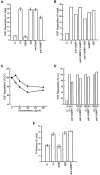
VAMP-7 antibodies block EGF breakdown. (A) NEM treatment blocks the degradation of EGF, but addition of IgG or anti–syntaxin 6 antibodies has no effect on the process. Addition of anti–VAMP-7 antibodies partially blocks degradation of EGF. Measurements were made three times and the standard deviation is indicated. An F-test indicated that the effect observed with anti–VAMP-7 antibodies was statistically significant compared with control antibodies (P < 0.01). (B) Anti–VAMP-7 affinity-purified polyclonal antibody also inhibits EGF breakdown. Including VAMP-7 protein but not GST reverses the inhibition of EGF degradation produced by the anti–VAMP-7 polyclonal antibody. VAMP-7 protein and GST alone have no effect in the assay. Measurements were made two times and the average is plotted. (C) Increasing amounts of VAMP-7 polyclonal or monoclonal antibodies result in an increased inhibition of EGF breakdown. Maximum inhibition is achieved at a concentration of 100 μg/ml VAMP-7 antibodies. Filled circles indicate polyclonal antibodies; open circles indicate mAbs. (D) EGF-loaded cells were chased for varying amounts of time at 37°C followed by SLO-permeabilization. Cells were then incubated with antibody and EGF degradation measured as described above. (E) Tf recycling is also reconstituted by addition of cytoplasm to SLO-permeabilized cells. Whereas NEM inhibits the recycling, neither IgG nor anti–VAMP-7 antibodies affect the process.
Similar articles
- Differential roles of syntaxin 7 and syntaxin 8 in endosomal trafficking.
Prekeris R, Yang B, Oorschot V, Klumperman J, Scheller RH. Prekeris R, et al. Mol Biol Cell. 1999 Nov;10(11):3891-908. doi: 10.1091/mbc.10.11.3891. Mol Biol Cell. 1999. PMID: 10564279 Free PMC article. - Syntaxin 7 and VAMP-7 are soluble N-ethylmaleimide-sensitive factor attachment protein receptors required for late endosome-lysosome and homotypic lysosome fusion in alveolar macrophages.
Ward DM, Pevsner J, Scullion MA, Vaughn M, Kaplan J. Ward DM, et al. Mol Biol Cell. 2000 Jul;11(7):2327-33. doi: 10.1091/mbc.11.7.2327. Mol Biol Cell. 2000. PMID: 10888671 Free PMC article. - Syntaxin 7 mediates endocytic trafficking to late endosomes.
Nakamura N, Yamamoto A, Wada Y, Futai M. Nakamura N, et al. J Biol Chem. 2000 Mar 3;275(9):6523-9. doi: 10.1074/jbc.275.9.6523. J Biol Chem. 2000. PMID: 10692457 - Membrane traffic to and from lysosomes.
Luzio JP, Pryor PR, Gray SR, Gratian MJ, Piper RC, Bright NA. Luzio JP, et al. Biochem Soc Symp. 2005;(72):77-86. doi: 10.1042/bss0720077. Biochem Soc Symp. 2005. PMID: 15649132 Review. - Multiple roles of the vesicular-SNARE TI-VAMP in post-Golgi and endosomal trafficking.
Chaineau M, Danglot L, Galli T. Chaineau M, et al. FEBS Lett. 2009 Dec 3;583(23):3817-26. doi: 10.1016/j.febslet.2009.10.026. Epub 2009 Oct 20. FEBS Lett. 2009. PMID: 19837067 Review.
Cited by
- The SNARE Vti1a-beta is localized to small synaptic vesicles and participates in a novel SNARE complex.
Antonin W, Riedel D, von Mollard GF. Antonin W, et al. J Neurosci. 2000 Aug 1;20(15):5724-32. doi: 10.1523/JNEUROSCI.20-15-05724.2000. J Neurosci. 2000. PMID: 10908612 Free PMC article. - VAMP7 modulates ciliary biogenesis in kidney cells.
Szalinski CM, Labilloy A, Bruns JR, Weisz OA. Szalinski CM, et al. PLoS One. 2014 Jan 22;9(1):e86425. doi: 10.1371/journal.pone.0086425. eCollection 2014. PLoS One. 2014. PMID: 24466086 Free PMC article. - Exploring the human genome with functional maps.
Huttenhower C, Haley EM, Hibbs MA, Dumeaux V, Barrett DR, Coller HA, Troyanskaya OG. Huttenhower C, et al. Genome Res. 2009 Jun;19(6):1093-106. doi: 10.1101/gr.082214.108. Epub 2009 Feb 26. Genome Res. 2009. PMID: 19246570 Free PMC article. - Endosomal trafficking of HIV-1 gag and genomic RNAs regulates viral egress.
Molle D, Segura-Morales C, Camus G, Berlioz-Torrent C, Kjems J, Basyuk E, Bertrand E. Molle D, et al. J Biol Chem. 2009 Jul 17;284(29):19727-43. doi: 10.1074/jbc.M109.019844. Epub 2009 May 18. J Biol Chem. 2009. PMID: 19451649 Free PMC article. - The WDR11 complex is a receptor for acidic-cluster-containing cargo proteins.
Deng H, Jia G, Li P, Tang Y, Zhao L, Yang Q, Zhao J, Wang J, Tu Y, Yong X, Zhang S, Mo X, Billadeau DD, Su Z, Jia D. Deng H, et al. Cell. 2024 Aug 8;187(16):4272-4288.e20. doi: 10.1016/j.cell.2024.06.024. Epub 2024 Jul 15. Cell. 2024. PMID: 39013469
References
- Advani R.J., Bae H.R., Bock J.B., Chao D.S., Doung Y.C., Prekeris R., Yoo J.S., Scheller R.H. Seven novel mammalian SNARE proteins localize to distinct membrane compartments. J. Biol. Chem. 1998;273:10317–10324. - PubMed
- Bennett M.K., Scheller R.H. A molecular description of synaptic vesicle membrane trafficking. Annu. Rev. Biochem. 1994;63:63–100. - PubMed
- Bennett M.K., Garcia-Arraras J.E., Elferink L.A., Peterson K., Fleming A.M., Hazuka C.D., Scheller R.H. The syntaxin family of vesicular transport receptors. Cell. 1993;74:863–873. - PubMed
- Bock J.B., Lin R.C., Scheller R.H. A new syntaxin family member implicated in targeting of intracellular transport vesicles. J. Biol. Chem. 1996;271:17961–17965. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous