Regulation of APC activity by phosphorylation and regulatory factors - PubMed (original) (raw)
Regulation of APC activity by phosphorylation and regulatory factors
S Kotani et al. J Cell Biol. 1999.
Retraction in
- Retraction.
Tanaka H, Yasuda H, Todokoro K. Tanaka H, et al. J Cell Biol. 2005 Apr 11;169(1):205. doi: 10.1083/jcb.199901060032505r. J Cell Biol. 2005. PMID: 15824138 Free PMC article. No abstract available.
Abstract
Ubiquitin-dependent proteolysis of Cut2/Pds1 and Cyclin B is required for sister chromatid separation and exit from mitosis, respectively. Anaphase-promoting complex/cyclosome (APC) specifically ubiquitinates Cut2/Pds1 at metaphase-anaphase transition, and ubiquitinates Cyclin B in late mitosis and G1 phase. However, the exact regulatory mechanism of substrate-specific activation of mammalian APC with the right timing remains to be elucidated. We found that not only the binding of the activators Cdc20 and Cdh1 and the inhibitor Mad2 to APC, but also the phosphorylation of Cdc20 and Cdh1 by Cdc2-Cyclin B and that of APC by Polo-like kinase and cAMP-dependent protein kinase, regulate APC activity. The cooperation of the phosphorylation/dephosphorylation and the regulatory factors in regulation of APC activity may thus control the precise progression of mitosis.
Figures
Figure 1
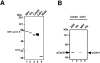
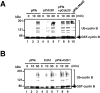
In vitro phosphorylation of Cdc20, Cdh1, and APC by MPF. (A) The Coomassie staining of the purified recombinant kinases, MPF (lane 1) and Plk (lane 2), and the recombinant substrates, Cdc20 (lane 3), Cdh1 (lane 4), and Mad2 (lane 5). (B) Human T7–tagged Cdc20 (lanes 1 and 2) or Cdh1 (lanes 3 and 4) was incubated with MPF (lanes 1 and 3) or Plk (lanes 2 and 4) and γ-[32P]ATP in a kinase buffer. The 32P-labeled Cdc20 and Cdh1 were immunoprecipitated by anti-T7 antibody and resolved in 7% SDS-PAGE. Numbers at left indicate the positions of molecular mass markers in kilodaltons. (C) In vitro phosphorylation of APC by MPF with or without Suc1/Cks1 (CksHs-1 or CksHs-2). In vitro kinase assays were performed in the presence of CksHs-1 (lanes 3 and 5), CksHs-2 (lanes 4 and 6), Plk (lanes 2, 5, and 6), MPF (lanes 1–6), and APC (lanes 1–6). Arrows indicate the labeled APC1/Tsg24, APC3/Cdc27, APC6/Cdc16, and an unidentified protein of 85 kD.
Figure 1
In vitro phosphorylation of Cdc20, Cdh1, and APC by MPF. (A) The Coomassie staining of the purified recombinant kinases, MPF (lane 1) and Plk (lane 2), and the recombinant substrates, Cdc20 (lane 3), Cdh1 (lane 4), and Mad2 (lane 5). (B) Human T7–tagged Cdc20 (lanes 1 and 2) or Cdh1 (lanes 3 and 4) was incubated with MPF (lanes 1 and 3) or Plk (lanes 2 and 4) and γ-[32P]ATP in a kinase buffer. The 32P-labeled Cdc20 and Cdh1 were immunoprecipitated by anti-T7 antibody and resolved in 7% SDS-PAGE. Numbers at left indicate the positions of molecular mass markers in kilodaltons. (C) In vitro phosphorylation of APC by MPF with or without Suc1/Cks1 (CksHs-1 or CksHs-2). In vitro kinase assays were performed in the presence of CksHs-1 (lanes 3 and 5), CksHs-2 (lanes 4 and 6), Plk (lanes 2, 5, and 6), MPF (lanes 1–6), and APC (lanes 1–6). Arrows indicate the labeled APC1/Tsg24, APC3/Cdc27, APC6/Cdc16, and an unidentified protein of 85 kD.
Figure 2
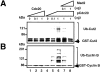
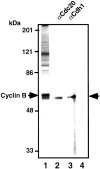
The pCdc20 but not Cdc20 activates ubiquitination of Cut2 and Cyclin B. In vitro ubiquitination assays were performed with purified mouse APC in S phase in the presence or absence of various amounts of Cdc20, pCdc20, and Mad2 as indicated. Cdc20 was phosphorylated by MPF and called pCdc20. The pCdc20 was purified by His-bind chromatography and washing with 0.5 M KCl to remove MPF. Activity to ubiquitinate GST-Cut2 (A) or GST-Cyclin B (B) was measured in the presence of recombinant E1 and hE2-C in the ubiquitination buffer. Reaction mixes were applied to 7% SDS-PAGE, and the polyubiquitinated GST-Cut2 and GST-Cyclin B were detected by immunoblotting with anti–Cyclin B antibody and anti–GST antibody, respectively. Polyubiquitinated GST-Cut2 and Cyclin B bands are shown as Ub-Cut2 and Ub-Cyclin B, respectively. Arrows indicate the positions of nonubiquitinated GST-Cut2 and GST-Cyclin B. Numbers at left indicate the positions of molecular mass marker in kilodaltons.
Figure 3
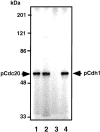
The Cdh1 but not pCdh1 activates ubiquitination of Cyclin B but not Cut2. In vitro ubiquitination assays were performed with purified mouse APC in S phase in the presence or absence of various amounts of Cdh1, pCdh1, and Mad2 as indicated. Cdh1 was phosphorylated by MPF, and purified by His-bind chromatography and washing with 0.5 M KCl to remove MPF. Activity to ubiquitinate GST-Cut2 (A) or GST-Cyclin B (B) was measured in the presence of recombinant E1 and hE2-C in the ubiquitination buffer. Reaction mixes were applied to 7% SDS-PAGE, and immunoblotted with anti–Cyclin B antibody and anti–GST antibody, respectively. Polyubiquitinated Cyclin B bands are shown as Ub-Cyclin B. Arrows indicate the positions of nonubiquitinated GST-Cut2 and GST-Cyclin B. Numbers at left indicate the positions of molecular mass marker in kilodaltons.
Figure 4
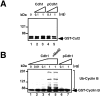
Suppression of pCdc20-, Cdh1-, or pPlk-induced APC activation by PKA phosphorylation. Purified APC was phosphorylated by pPlk and/or PKA, and Cdc20 was phosphorylated by MPF. APC phosphorylated by pPlk and/or PKA (indicated by +) was incubated with GST-Cut2 (top panel) or human GST-Cyclin B (bottom panel) in the presence (+) or absence (no mark) of pCdc20 and Cdh1 in the ubiquitination buffer at 25°C for 30 min. Samples were applied to 7% SDS-PAGE and ubiquitinated GST-Cyclin B or GST-Cut2 was detected by immunoblotting with anti–Cyclin B1 antibody and anti–GST antibody, respectively, and visualized by the enhanced chemiluminescence reaction.
Figure 5
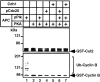
pPlk synergistically acts on APC activation with pCdc20 or Cdh1. (A) pPlk synergistically acts on APC activation with pCdc20. Purified APC was phosphorylated by pPlk, and Cdc20 was phosphorylated by MPF. The phosphorylated (lanes 1–3 and 7–9) or untreated APC (lanes 4–6) was incubated with GST-Cyclin in the presence (lanes 4–9) or absence (lanes 1–3) of pCdc20 in the ubiquitination buffer at 25°C for 0, 10, and 30 min. Ubiquitinated GST-Cyclin B was detected by immunoblotting with anti–Cyclin B1 antibody. (B) pPlk synergistically functions with Cdh1 in APC activation. The same experiments described in A were done using Cdh1 in place of pCdc20.
Figure 6
Binding of Cdc20, pCdc20, and Cdh1 to APC and pAPC. (A) Purified APC in S phase, which was prepared by immunoprecipitation with anti–Cdc27 antibody, was treated with pPlk (lanes 7–12), or PKA (lanes 13–16), or without treatment (lanes 1–6). Phosphorylated or untreated APC was incubated with T7-tagged human Cdc20 (lanes 1, 3, 7, 9, and 13), pCdc20 (lanes 2, 4, 8, 10, and 14), Cdh1 (lanes 5, 11, and 15), or pCdh1 (lanes 6, 12, and 16), in the presence (lanes 3, 4, 9, and 10) or absence of Mad2, washed well, and the proteins bound to APC were resolved by 7% SDS-PAGE. The bound proteins were detected by immunoblotting with anti-T7 antibody. (B) The purified S phase APC was incubated with Cdc2-GST-Cyclin B and γ-[32P]ATP in the presence of Cdc20 (lanes 1 and 3) or Cdh1 (lane 2), and the 32P-labeled proteins were immunoprecipitated by anti–Cdc27 antibody (lanes 1 and 2) or by preimmune antibody (lane 3).
Figure 6
Binding of Cdc20, pCdc20, and Cdh1 to APC and pAPC. (A) Purified APC in S phase, which was prepared by immunoprecipitation with anti–Cdc27 antibody, was treated with pPlk (lanes 7–12), or PKA (lanes 13–16), or without treatment (lanes 1–6). Phosphorylated or untreated APC was incubated with T7-tagged human Cdc20 (lanes 1, 3, 7, 9, and 13), pCdc20 (lanes 2, 4, 8, 10, and 14), Cdh1 (lanes 5, 11, and 15), or pCdh1 (lanes 6, 12, and 16), in the presence (lanes 3, 4, 9, and 10) or absence of Mad2, washed well, and the proteins bound to APC were resolved by 7% SDS-PAGE. The bound proteins were detected by immunoblotting with anti-T7 antibody. (B) The purified S phase APC was incubated with Cdc2-GST-Cyclin B and γ-[32P]ATP in the presence of Cdc20 (lanes 1 and 3) or Cdh1 (lane 2), and the 32P-labeled proteins were immunoprecipitated by anti–Cdc27 antibody (lanes 1 and 2) or by preimmune antibody (lane 3).
Figure 7
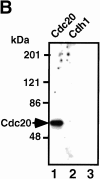
MPF phosphorylates Cdc20 and Cdh1 during mitosis in vivo. The immunoprecipitates of anti–Cyclin B antibody from mitotic K562 cell extracts were incubated with γ-[32P]ATP, washed with 1% SDS, diluted with the kinase buffer, and the labeled Cdc20 (lane 2) and Cdh1 (lane 3) were immunoprecipitated with each specific antibody. Lane 1 shows the total 32P-labeled proteins in the immunoprecipitates. Lane 4 shows the immunoprecipitates with preimmune antibody. Right arrow indicates the 32P-labeled Cdc20 and Cdh1. Left arrow shows 32P-labeled Cyclin B.
Figure 8
Interaction of pCdc20 and Cdh1 with APC during mitosis in vivo. K562 cells were labeled with 32P-orthophosphate during mitosis, and the APC was purified by Resource Q chromatography and immunoprecipitation with anti–Cdc27 antibody. The 32P-labeled Cdc20 was immunoprecipitated with anti–Cdc20 antibody from this purified APC (lane 1) and from the total cell lysates (lane 2). The 32P-labeled Cdh1 was immunoprecipitated with its specific antibody from the purified APC (lane 3) and from the total cell lysates (lane 4). Arrows indicate pCdc20 and pCdh1 phosphorylated in vivo.
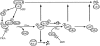
Figure 9
Model of regulation of APC activity.
Similar articles
- Yeast Hct1 recognizes the mitotic cyclin Clb2 and other substrates of the ubiquitin ligase APC.
Schwab M, Neutzner M, Möcker D, Seufert W. Schwab M, et al. EMBO J. 2001 Sep 17;20(18):5165-75. doi: 10.1093/emboj/20.18.5165. EMBO J. 2001. PMID: 11566880 Free PMC article. - PKA and MPF-activated polo-like kinase regulate anaphase-promoting complex activity and mitosis progression.
Kotani S, Tugendreich S, Fujii M, Jorgensen PM, Watanabe N, Hoog C, Hieter P, Todokoro K. Kotani S, et al. Mol Cell. 1998 Feb;1(3):371-80. doi: 10.1016/s1097-2765(00)80037-4. Mol Cell. 1998. PMID: 9660921 - Chromosome separation and exit from mitosis in budding yeast: dependence on growth revealed by cAMP-mediated inhibition.
Anghileri P, Branduardi P, Sternieri F, Monti P, Visintin R, Bevilacqua A, Alberghina L, Martegani E, Baroni MD. Anghileri P, et al. Exp Cell Res. 1999 Aug 1;250(2):510-23. doi: 10.1006/excr.1999.4531. Exp Cell Res. 1999. PMID: 10413604 - Subunits and substrates of the anaphase-promoting complex.
Peters JM. Peters JM. Exp Cell Res. 1999 May 1;248(2):339-49. doi: 10.1006/excr.1999.4443. Exp Cell Res. 1999. PMID: 10222126 Review. - Control of metaphase-anaphase progression by proteolysis: cyclosome function regulated by the protein kinase A pathway, ubiquitination and localization.
Yanagida M, Yamashita YM, Tatebe H, Ishii K, Kumada K, Nakaseko Y. Yanagida M, et al. Philos Trans R Soc Lond B Biol Sci. 1999 Sep 29;354(1389):1559-69; discussion 1569-70. doi: 10.1098/rstb.1999.0499. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10582241 Free PMC article. Review.
Cited by
- Rhynchosia volubilis Promotes Cell Survival via cAMP-PKA/ERK-CREB Pathway.
Ahn SH, Suh JS, Jang YK, Kim HS, Choi GH, Kim E, Kim TJ. Ahn SH, et al. Pharmaceuticals (Basel). 2022 Jan 6;15(1):73. doi: 10.3390/ph15010073. Pharmaceuticals (Basel). 2022. PMID: 35056130 Free PMC article. - Ordered dephosphorylation initiated by the selective proteolysis of cyclin B drives mitotic exit.
Holder J, Mohammed S, Barr FA. Holder J, et al. Elife. 2020 Sep 1;9:e59885. doi: 10.7554/eLife.59885. Elife. 2020. PMID: 32869743 Free PMC article. - CKS1 Germ Line Exclusion Is Essential for the Transition from Meiosis to Early Embryonic Development.
Ellederova Z, Del Rincon S, Koncicka M, Susor A, Kubelka M, Sun D, Spruck C. Ellederova Z, et al. Mol Cell Biol. 2019 Jun 13;39(13):e00590-18. doi: 10.1128/MCB.00590-18. Print 2019 Jul 1. Mol Cell Biol. 2019. PMID: 30988159 Free PMC article. - Erratum to: Controlling the response to DNA damage by the APC/C-Cdh1.
de Boer HR, Llobet SG, van Vugt MA. de Boer HR, et al. Cell Mol Life Sci. 2016 Aug;73(15):2985-2998. doi: 10.1007/s00018-016-2279-x. Cell Mol Life Sci. 2016. PMID: 27251328 Free PMC article. No abstract available. - A decade of the anaphase-promoting complex in the nervous system.
Huang J, Bonni A. Huang J, et al. Genes Dev. 2016 Mar 15;30(6):622-38. doi: 10.1101/gad.274324.115. Genes Dev. 2016. PMID: 26980187 Free PMC article. Review.
References
- Amon A., Irniger S., Nasmyth K. Closing the cell cycle circle in yeastG2 cyclin proteolysis initiated at mitosis persists until the activation of G1 cyclins in the next cycle. Cell. 1994;77:1037–1050. - PubMed
- Charles J.F., Jaspersen S.L., Tinker-Kulberg R.L., Hwang L., Szidon A., Morgan D.O. The Polo-related kinase Cdc5 activates and is destroyed by the mitotic cyclin destruction machinery in S. cerevisiae . Curr. Biol. 1998;8:497–507. - PubMed
- Clute P., Pines J. Temporal and spatial control of cyclin B1 destruction in metaphase. Nat. Cell Biol. 1999;1:82–87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous