Nuclear export of the small ribosomal subunit requires the ran-GTPase cycle and certain nucleoporins - PubMed (original) (raw)
Nuclear export of the small ribosomal subunit requires the ran-GTPase cycle and certain nucleoporins
T I Moy et al. Genes Dev. 1999.
Abstract
After their assembly in the nucleolus, ribosomal subunits are exported from the nucleus to the cytoplasm. After export, the 20S rRNA in the small ribosomal subunit is cleaved to yield 18S rRNA and the small 5' ITS1 fragment. The 5' ITS1 RNA is normally degraded by the cytoplasmic Xrn1 exonuclease, but in strains lacking XRN1, the 5' ITS1 fragment accumulates in the cytoplasm. Using the cytoplasmic localization of the 5' ITS1 fragment as an indicator for the export of the small ribosomal subunit, we have identified genes that are required for ribosome export. Ribosome export is dependent on the Ran-GTPase as mutations in Ran or its regulators caused 5' ITS1 to accumulate in the nucleoplasm. Mutations in the genes encoding the nucleoporin Nup82 and in the NES exporter Xpo1/Crm1 also caused the nucleoplasmic accumulation of 5' ITS1. Mutants in a subset of nucleoporins and in the nuclear transport factors Srp1, Kap95, Pse1, Cse1, and Mtr10 accumulate the 5' ITS1 in the nucleolus and affect ribosome assembly. In contrast, we did not detect nuclear accumulation of 5' ITS1 in 28 yeast strains that have mutations in other genes affecting nuclear trafficking.
Figures
Figure 1
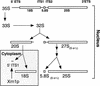
Diagram of rRNA processing in S. cerevisiae. 18S, 5.8S, and 25S rRNAs are transcribed as a 35S pre-rRNA in the nucleolus. The mature forms of the rRNAs are depicted as open bars and the external transcribed spacers (ETSs) and ITSs are depicted as lines. The 5′ ETS undergoes endonucleolytic cleavages to yield the 33S pre-rRNA and then the 32S pre-rRNA. The ITS1 in the 32S pre-rRNA is cleaved to produce the 20S rRNA and the 27S rRNA. The 27S rRNA is processed through one of two pathways to produce the mature 5.8S and 25S rRNAs. The different pathways produce 5.8S rRNAs with different lengths (long or short). The final maturation of the 20S rRNA occurs in the cytoplasm. In the cytoplasm, an endonucleolytic cleavage produces the mature 18S rRNA and a 209- to 210-base fragment, the 5′ ITS1. The 5′ ITS1 fragment is then degraded by the exonuclease XRN1 (for review, see Venema and Tollervey 1995).
Figure 2
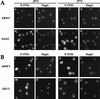
Localization of 5′ ITS1 RNA in wild-type (XRN1) and Δxrn1 yeast cells. Chromosomal DNA was labeled with DAPI to identify the location of the nucleus. DAPI-stained cells are shown in blue (a,e). 5′ ITS1 RNA was hybridized and labeled with a 200-bp probe and Texas Red-conjugated antibodies and is shown in red (b,f). Immunofluorescence with anti-Nop1 was used to identfy the location of the nucleolus and is shown in green (c,g). The DAPI, 5′ ITS1, and Nop1 signals were overlayed in d and h; colocalization of the 5′ ITS1 and Nop1 produces a yellow signal.
Figure 3
Localization of 5′ ITS1 and Nop1 in wild-type strains and rRNA processing mutants. Localization was determined as in Fig. 2. (A) XRN1 and Δxrn1 cells were grown at 25°C or shifted to 37°C for 1 hr. (B) mtr4-1 Δxrn1 and fal1-1 Δxrn1 cells were grown at 25°C or shifted to 37°C for 1 or 4 hr, respectively.
Figure 4
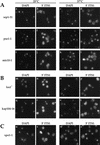
Localization of 5′ ITS1 in mutants affecting the Ran–GTPase cycle. (A) 5′ ITS1 was localized in prp20-1 (RanGEF), rna1-1 (RanGAP), and yrb1-1 (RanBP1) cells grown at permissive (25°C) or shifted to restrictive (37°C) temperature for 1 hr (b,e,h,k,n,q). The cells shown in this figure lack the Xrn1 exonuclease. DAPI staining is used to identify the location of chromosomal DNA (a,d,g,j,m,p) and Nomarski optics are used to visualize cell morphology (c,f,i,l,o,r). 5′ ITS1 localization to the entire nucleus of yrb1-1 cells (arrows) and to the nucleolus (arrowheads) is indicated in q. (B) Localization of 5′ ITS1 and Nop1 in prp20-1 Δxrn1 cells shifted to 37°C. The images were deconvolved and the three dimensional reconstructions were projected onto two dimensions. 5′ ITS1 signal is shown in red (a) and Nop1 signal is shown in green (b). The 5′ ITS1 and Nop1 signals were overlayed in three dimensions and are shown in c.
Figure 4
Localization of 5′ ITS1 in mutants affecting the Ran–GTPase cycle. (A) 5′ ITS1 was localized in prp20-1 (RanGEF), rna1-1 (RanGAP), and yrb1-1 (RanBP1) cells grown at permissive (25°C) or shifted to restrictive (37°C) temperature for 1 hr (b,e,h,k,n,q). The cells shown in this figure lack the Xrn1 exonuclease. DAPI staining is used to identify the location of chromosomal DNA (a,d,g,j,m,p) and Nomarski optics are used to visualize cell morphology (c,f,i,l,o,r). 5′ ITS1 localization to the entire nucleus of yrb1-1 cells (arrows) and to the nucleolus (arrowheads) is indicated in q. (B) Localization of 5′ ITS1 and Nop1 in prp20-1 Δxrn1 cells shifted to 37°C. The images were deconvolved and the three dimensional reconstructions were projected onto two dimensions. 5′ ITS1 signal is shown in red (a) and Nop1 signal is shown in green (b). The 5′ ITS1 and Nop1 signals were overlayed in three dimensions and are shown in c.
Figure 5
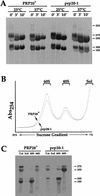
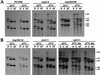
rRNA processing of wild-type (PRP20) and prp20-1 cells. (A) rRNA processing by pulse-chase analysis. PRP20 and prp20-1 cells were grown at permissive temperatures or shifted to 37°C for 10 min. Cells were pulse-labeled and chased for 0, 3, or 10 min. RNA was extracted and run on a formaldehyde–agarose gel. The time points of the chase are labeled above each lane. (B) Fractionation of ribosomal subunits by sucrose gradient centrifugation. PRP20 and prp20-1 cells were shifted to 37°C for 10 min. _A_254 nm was used to detect the peaks of the 60S and 40S ribosomal subunits. Fractions for the 60S, 40S, and soluble proteins were collected. (C) rRNA present in the 60S, 40S, and soluble fractions were separated on formaldehyde–agarose gels. Total rRNA is also shown.
Figure 6
Localization of 5′ ITS1 in nucleoporin mutants. DAPI staining is shown in a,c,e,g,i,k,m,o,q,s,u,w and 5′ ITS1 localization is shown in b,d,f,h,j,l,n,p,r,t,v,x. (A) Nucleoporin mutants that acccumulate 5′ ITS1 in the nucleolus at restrictive temperatures. _nup2-4 Δxrn1, nup84_− Δxrn1, and rat3-1/nup120 Δxrn1 cells were grown at permissive temperatures or shifted to 37°C for 3 hr, 3 hr, or 1 hr, respectively. (B) Nucleoporin mutants that do not accumulate the 5′ ITS1 in the nucleus. gle2/nup40 Δxrn1 and rat7/nup159 Δxrn1 cells were grown at 25°C or shifted to 37°C for 1 hr. (C) 5′ ITS1 localization in nup82Δ108 Δxrn cells grown at 25°C or shifted to 37°C for 4 hr. About a third of nup82Δ108 Δxrn1 cells accumulate the 5′ ITS1 in their entire nucleus.
Figure 7
rRNA processing of nucleoporin mutants and importin β mutants by pulse-chase analysis. (A) Wild-type (PSY580), nup2-4, and nup82Δ108 strains were grown at permissive temperatures or shifted to 37°C for 3, 3, or 4 hr, respectively. (B) kap104-16, pse1-1, and xpo1-1 strains were grown at permissive temperatures or shifted to 37°C for 3 hr, 5 hr, or the times indicated.
Figure 8
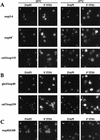
Localization of 5′ ITS1 in nuclear transport factor mutants. DAPI staining is shown in a,c,e,g,i,k,m,o,q,s,u,w and 5′ ITS1 localization is shown in b,d,f,h,j,l,n,p,r,t,v,x. (A) Importin α mutant srp1-31 Δxrn1 and importin β mutants pse1-1 Δxrn1 and mtr10-1 Δxrn1 were grown at permissive temperatures or shifted to 37°C for 3, 5, or 3 hr, respectively. These strains accumulate 5′ ITS1 in the nucleolus at restrictive temperatures. (B) β Mutants Δlos1 Δxrn1 and kap104-16 Δxrn1 do not accumulate 5′ ITS1 in their nucleus when shifted to 37°C for 4 hr. (C) xpo1-1 Δxrn1 cells accumulates 5′ ITS1 in their entire nucleus when shifted to 37°C for 4 hr.
Figure 9
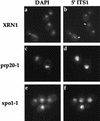
Localization of 5′ ITS1 in strains possessing functional XRN1 exonuclease. Wild-type (XRN1), prp20-1, and xpo1-1 cells were shifted to 37°C to induce mutant phenotypes. 5′ ITS1 was localized to the nucleolus (arrowheads) of wild-type cells at restrictive temperatures. 5′ ITS was localized to the entire nucleus of prp20-1 and xpo1-1 cells, shifted to 37°C.
Similar articles
- Requirements for the nuclear export of the small ribosomal subunit.
Moy TI, Silver PA. Moy TI, et al. J Cell Sci. 2002 Jul 15;115(Pt 14):2985-95. doi: 10.1242/jcs.115.14.2985. J Cell Sci. 2002. PMID: 12082158 - Factors affecting nuclear export of the 60S ribosomal subunit in vivo.
Stage-Zimmermann T, Schmidt U, Silver PA. Stage-Zimmermann T, et al. Mol Biol Cell. 2000 Nov;11(11):3777-89. doi: 10.1091/mbc.11.11.3777. Mol Biol Cell. 2000. PMID: 11071906 Free PMC article. - Ultrastructural localization of rRNA shows defective nuclear export of preribosomes in mutants of the Nup82p complex.
Gleizes PE, Noaillac-Depeyre J, Léger-Silvestre I, Teulières F, Dauxois JY, Pommet D, Azum-Gelade MC, Gas N. Gleizes PE, et al. J Cell Biol. 2001 Dec 10;155(6):923-36. doi: 10.1083/jcb.200108142. Epub 2001 Dec 10. J Cell Biol. 2001. PMID: 11739405 Free PMC article. - Nuclear export of ribosomal subunits.
Johnson AW, Lund E, Dahlberg J. Johnson AW, et al. Trends Biochem Sci. 2002 Nov;27(11):580-5. doi: 10.1016/s0968-0004(02)02208-9. Trends Biochem Sci. 2002. PMID: 12417134 Review. - Review: transport of tRNA out of the nucleus-direct channeling to the ribosome?
Grosshans H, Simos G, Hurt E. Grosshans H, et al. J Struct Biol. 2000 Apr;129(2-3):288-94. doi: 10.1006/jsbi.2000.4226. J Struct Biol. 2000. PMID: 10806079 Review.
Cited by
- Deletions in the S1 domain of Rrp5p cause processing at a novel site in ITS1 of yeast pre-rRNA that depends on Rex4p.
Eppens NA, Faber AW, Rondaij M, Jahangir RS, van Hemert S, Vos JC, Venema J, Raué HA. Eppens NA, et al. Nucleic Acids Res. 2002 Oct 1;30(19):4222-31. doi: 10.1093/nar/gkf538. Nucleic Acids Res. 2002. PMID: 12364601 Free PMC article. - Uncoupling ribosome biogenesis regulation from RNA polymerase I activity during herpes simplex virus type 1 infection.
Belin S, Kindbeiter K, Hacot S, Albaret MA, Roca-Martinez JX, Thérizols G, Grosso O, Diaz JJ. Belin S, et al. RNA. 2010 Jan;16(1):131-40. doi: 10.1261/rna.1935610. Epub 2009 Nov 24. RNA. 2010. PMID: 19934231 Free PMC article. - Prefabrication of a ribosomal protein subcomplex essential for eukaryotic ribosome formation.
Peña C, Schütz S, Fischer U, Chang Y, Panse VG. Peña C, et al. Elife. 2016 Dec 8;5:e21755. doi: 10.7554/eLife.21755. Elife. 2016. PMID: 27929371 Free PMC article. - Regulation of ribosome biogenesis by nucleostemin 3 promotes local and systemic growth in Drosophila.
Hartl TA, Ni J, Cao J, Suyama KL, Patchett S, Bussiere C, Gui DY, Tang S, Kaplan DD, Fish M, Johnson AW, Scott MP. Hartl TA, et al. Genetics. 2013 May;194(1):101-15. doi: 10.1534/genetics.112.149104. Epub 2013 Feb 22. Genetics. 2013. PMID: 23436180 Free PMC article. - Late cytoplasmic maturation of the small ribosomal subunit requires RIO proteins in Saccharomyces cerevisiae.
Vanrobays E, Gelugne JP, Gleizes PE, Caizergues-Ferrer M. Vanrobays E, et al. Mol Cell Biol. 2003 Mar;23(6):2083-95. doi: 10.1128/MCB.23.6.2083-2095.2003. Mol Cell Biol. 2003. PMID: 12612080 Free PMC article.
References
- Adams A, Gottschling DE, Kaiser CA, Stearns T. Methods in yeast genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997.
- Aebi M, Clark MW, Vijayraghavan U, Abelson J. A yeast mutant, PRP20, altered in mRNA metabolism and maintenance of the nuclear structure, is defective in a gene homologous to the human gene RCC1 which is involved in the control of chromosome condensation. Mol & Gen Genet. 1990;224:72–80. - PubMed
- Agard DA, Hiraoka Y, Shaw P, Sedat JW. Fluorescence microscopy in three dimensions. Methods Cell Biol. 1989;30:353–377. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous