A role for the Cdc7 kinase regulatory subunit Dbf4p in the formation of initiation-competent origins of replication - PubMed (original) (raw)
A role for the Cdc7 kinase regulatory subunit Dbf4p in the formation of initiation-competent origins of replication
P Pasero et al. Genes Dev. 1999.
Abstract
Using a reconstituted DNA replication assay from yeast, we demonstrate that two kinase complexes are essential for the promotion of replication in vitro. An active Clb/Cdc28 kinase complex, or its vertebrate equivalent, is required in trans to stimulate initiation in G(1)-phase nuclei, whereas the Dbf4/Cdc7 kinase complex must be provided by the template nuclei themselves. The regulatory subunit of Cdc7p, Dbf4p, accumulates during late G(1) phase, becomes chromatin associated prior to Clb/Cdc28 activation, and assumes a punctate pattern of localization that is similar to, and dependent on, the origin recognition complex (ORC). The association of Dbf4p with a detergent-insoluble chromatin fraction in G(1)-phase nuclei requires ORC but not Cdc6p or Clb/Cdc28 kinase activity, and correlates with competence for initiation. We propose a model in which Dbf4p targets Cdc7p to the prereplication complex prior to the G(1)/S transition, by a pathway parallel to, but independent of, the Cdc6p-dependent recruitment of MCMs.
Figures
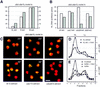
Figure 1
Cdc28p, but not Cdc7p, is required in trans to activate G1 nuclei. (A) G1-phase nuclei prepared from clb5,6Δ cells (GA-769) arrested with α-factor were used as template for in vitro replication in the presence of the indicated nuclear extracts. All reactions are performed in parallel with the same template nuclei. The extract labeled G1 is prepared from pre-Start cln1 cln2 cln3 GAL–CLN3 cells (GA-719, see Materials and Methods); (S) A wild-type GA-59 strain synchronized in S phase by α-factor block release; (M) cdc16-1 cells (GA-161) arrested in mitosis by a shift to 37°C after α-factor synchronization. Replication was performed for 2 hr at 30°C in the presence of BrdUTP and [α-32P]dCTP as described in Materials and Methods. The peak of semiconservative DNA replication (HL DNA) was integrated and converted to pmols on the basis of the specific activity of the incorporated dCTP (dark gray bars). The H1 kinase activity (light gray bars) was quantitated for a standard amount of extract, with the mitotic extracts as an arbitrary 100% (see Materials and Methods). Identical results were obtainedwith CLB5CLB6 nuclei synchronized by α-factor block as template (see D,E). (B) G1 nuclei from GA-769 cells (clb5clb6) arrested with α-factor were used in replication reactions at either 23°C (light gray bars) or 35°C (dark gray bars) with wild-type (GA-59), cdc7-1 (GA-85), cdc28-4 (GA-112), or dbf4 (GA-849) S-phase extracts prepared as described in Materials and Methods. Incorporation into HL DNA was quantitated as in A. Extracts from cdc7-4 mutants prepared at either 23°C or 37°C gave results identical to those shown here for cdc7-1 (GA-85). (C) The same reactions performed in B were carried out in the presence of DIG–dUTP instead of BrdUTP. The sites of incorporation were revealed by confocal microscopy of α-DIG–FITC (yellow signal; Boehringer Mannheim) superimposed on genomic DNA (red). Bar, 2 μm. (D) Wild-type G1 nuclei prepared from α-factor arrested GA-850 cells were allowed to incorporate BrdUTP as described in A, in a G1-phase extract prepared from GA-851 cells (cdc4-1) arrested for 4 hr at 37°C. A total of 10 units of baculovirus-expressed Xenopus CycB/Cdc2 kinase was either added (●) or not (○) prior to the reaction. Shown is the profile of the [α-32P]dCTP incorporated into genomic DNA after separation on a CsCl gradient; (HL) buoyant density of fully substituted DNA; (LL) buoyant density of unsubstituted DNA. (E) Replication assay with the same G1 nuclei as in D incubated in a wild-type S-phase extract (GA-59; ●), in buffer alone (○), or buffer supplemented with 10 units of CycB/Cdc2 kinase (▴). Slightly less stimulation was observed on addition of an excess of recombinant human CycE/Cdk2 or Clb5/Cdc28 (data not shown).
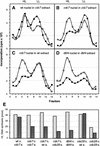
Figure 2
The Dbf4/Cdc7 complex is required in cis for the initiation of DNA replication. (A) In vitro DNA replication of wild-type G1-phase nuclei (GA-850) was performed as in Fig. 1A at either 23°C (○) or 35°C (●), in a cdc7-1 (GA-85) S-phase extract from cells arrested at 37°C. Shown are profiles of the [α-32P]dCTP incorporated after buoyant density gradient separation. HL and LL are as in Fig. 1D. The increase in LL DNA may reflect elevated repair or nuclease activities at 35°C. (B) Profile after buoyant density gradient separation of the [α-32P]dCTP incorporated into cdc7-1 G1-phase nuclei (GA-366), prepared at permissive temperature after α-factor arrest, and incubated in the same cdc7-1 S-phase extract as in A. Replication was performed at either 23°C (○) or 35°C (●). (C) Profile of [α-32P]dCTP incorporation into the same cdc7-1 G1-phase nuclei as in B incubated in a wild-type (GA-59) S-phase extract. (D) Profile after buoyant density gradient separation of the [α-32P]dCTP incorporated into dbf4 (GA-849) G1-phase nuclei isolated from cells arrested with α-factor at permissive temperature, and incubated in a dbf4 (GA-849) S-phase extract prepared by α-factor arrest release at permissive temperature (see Materials and Methods). Replication was carried out for 1 hr at either 23°C (○) or 35°C (●). (E) The peak of HL DNA synthesis was integrated for the reactions shown in A–D and converted to pmoles of DNA synthesized on the basis of the specific activity of [α-32P]dCTP. Parallel reactions were performed with G1-phase nuclei from cdc7-1 (GA-366) and cdc28-4 (GA-112) cells isolated after α-factor arrest. These were incubated at 23°C (light gray bars) and 35°C (dark gray bars) in either a wild-type (GA-59) or a cdc28-4 (GA-112) S-phase extract, and demonstrate that _cdc28_-deficient nuclei can be complemented in trans.
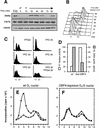
Figure 3
Initiation of DNA replication in vitro depends on the presence of Dbf4p in the template G1 nuclei. (A) Dbf4p levels fluctuate during the cell cycle. A culture of GA-850 cells (carrying a Myc-tagged DBF4 gene) were arrested in G1 phase for 90 min with α-factor. Total protein extracts were prepared from the exponential culture (Exp), after α-factor arrest (αF) and every 15 min after release from the block. After separation by SDS-PAGE, fractions were blotted with either 9E10 Mab (Myc epitope), a polyclonal antibody against Cdc7p (gift of C. Mann, Institute of Atomic Energy Research, Saclay, France) or TAT1 Mab (anti-tubulin) as a loading control. (B) FACS analysis of the DNA content of GA-850 cells during the α-factor block-release experiment shown in A. (C) GA-896 cells that have a unique copy of DBF4 under the control of a galactose-inducible promoter, were grown exponentially on galactose-containing medium (YPGal) or arrested for 2, 3, 4, or 5 hr on glucose-containing medium (YPD). DNA content analysis by FACS indicates arrest in G1 after 2 hr, and a peak at 0.5C corresponds to cells undergoing a reductional anaphase in the absence of Dbf4p after 3 hr on YPD. One fraction of the culture was shifted back to YPGal after 3 hr on YPD (YPD 3 hr, YPGal 2 hr) and FACS analysis shows that a significant proportion of the cells are able to enter S phase. (D) Nuclei were prepared from GA-850 cells arrested with α-factor (wt) or from GA-896 cells arrested at the G1/S transition due to a lack of Dbf4p expression after 2.5 hr on YPD (labeled Gal–DBF4). H1 kinase activity was monitored as described in Materials and Methods. We see low Clb/Cdc28 kinase activity in our G1-phase nuclei, and high kinase activity in the Dbf4p-depleted nuclei. Their ability to support semiconservative replication was monitored in standard reactions in a wild-type S-phase extract (GA-59; see F). (Dark gray bars) The integrated HL peak. (E,F) Profiles of the radioactivity recovered after buoyant density gradient analysis are shown for GA-850 G1 nuclei (E) or for Dbf4p-depleted nuclei (GA-896, F), incubated under standard conditions either with a wild-type S-phase extract (GA-59, ●) or without added extract (○). HL and LL DNA migrate in fractions 2–5 and 8–10, respectively.
Figure 4
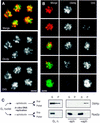
A fraction of Dbf4p localizes to subnuclear foci. (A–C) Exponentially growing cells were fixed with 4% paraformaldehyde in YPD and were analyzed by immunofluorescence, as described in Materials and Methods. Dbf4p–Myc and Orc2–Myc were detected in the strains GA-850 and GA-893, respectively, with mAb 9E10. Clb5–HA was detected in GA-453 cells with mAb 12AC5. mAbs are detected by a DTAF-coupled goat anti-mouse secondary antibody. The background has been intentionally raised to show the contour of the cells so that the stage of the cell cycle can be deduced. In all cases, the mAb and secondary antibodies gave no significant signal for cells with no tagged construct. (Arrowheads) Mitotic cells. (D,E) Dbf4p–Myc and Orc1p–HA were detected in diploid GA-1123 cells by sequential incubation of the fixed spheroplasts with 9E10 (anti-Myc) and a Cy5-conjugated secondary antibody, followed by incubation with a rabbit serum specific for the HA epitope (Santa Cruz Biotechnology) and a DTAF-conjugated secondary antibody. Staining patterns were mutually exclusive when both primary antibodies were used at once (see text). (F) Merge of the signals in D and E, Dbf4p–Myc, and Orc1p–HA are in red and green, respectively, with the region of overlap in yellow. (G,H) Punctate pattern of Dbf4–Myc is altered in orc2-1 cells. Exponential cultures of GA-850 cells (ORC2, G) and GA-1027 cells (orc2-1, H) were grown at 23°C and fixed. Dbf4p–Myc was detected by immunofluorescence as described in A. Typical fields of cells are shown. The cell cycle fluctuation in Dbf4p levels can be observed in these nonsynchronous populations. Noteworthy is the decrease in the punctate appearance of the Dbf4p signal in the orc2-1 strain that is otherwise isogenic to GA-850. Bars, 2 μm.
Figure 5
Dbf4p cofractionates with ORC in a scaffold fraction and associates with a chromatin pellet in an ORC-dependent manner. (A) The association of Dbf4p and other proteins with an insoluble nuclear fraction was analyzed basically as described in Donovan et al. (1997) and Liang and Stillman (1997). Spheroplasts prepared from nonsynchronized cells were lysed in a nonionic detergent. The insoluble nuclear fraction (P) was separated from the soluble proteins (S) by low-speed centrifugation and was resuspended in an equal volume as the supernatant. In some experiments, the pellet was further digested with DNase I and the solubilized chromatin fraction (Chr) was separated from the insoluble nuclear scaffold (Sc) by low speed centrifugation (see Materials and Methods). (B) Chromatin assays were performed on exponentially growing cultures of GA-850 (Dbf4–Myc) and GA-893 (Orc2–Myc). The antibodies used for Western blot analysis of total protein extracts (T), supernatant (S), and insoluble nuclear (P) fractions are described in Fig. 3 and in Materials and Methods. Mab414 (Berkeley Antibody Inc., CA) detects the nuclear pore proteins Nup100, Nup116 and Nup145, of which the central band (Nup116, indicated with bar) reproducibly partitions equally between soluble and insoluble nuclear fractions, providing an internal control for loading. In this panel the strain GA-893 (Orc2–Myc) was used for the detection of Orc2p. (C) Insoluble nuclear fraction from an exponentially growing culture of GA-850 cells (Dbf4–Myc) was further fractionated by DNase I digestion. Very little protein is recovered in the final scaffold pellet and therefore three times the amount was loaded to facilitate quantitation. Orc2p is detected with anti-Orc2p (JAb12, gift of J. Diffley), Mcm2p is detected with a goat polyclonal serum, YN-19, (Santa Cruz Antibodies, CA) and Dbf4–Myc with 9E10 on identical scaffold (Sc) and soluble chromatin (Chr) samples. Quantitation of protein distribution between the two fractions is shown on the graph at right. (D) Isogenic wild-type (GA-850) and orc2-1 (GA-1027) cells were arrested in G1 phase at 23°C with α-factor, were incubated at 37°C for 30 min in the presence of α-factor and were released from the block at 37°C for 60 min. After spheroplasting, permeabilization of the cells with Triton X-100 and centrifugation, the amount of Dbf4p in S and P fractions was quantitated by immunoblotting in three independent experiments. One-tenth of the supernatant was loaded on the gel relative to the pellet fraction. Nucleoporins (Nup) were used as loading control and for normalization. (E) FACS analysis of a typical experiment in which orc2-1 and wild-type cells were arrested with α-factor at permissive temperature (αF) and released for 60 min at 37°C (rel.).
Figure 5
Dbf4p cofractionates with ORC in a scaffold fraction and associates with a chromatin pellet in an ORC-dependent manner. (A) The association of Dbf4p and other proteins with an insoluble nuclear fraction was analyzed basically as described in Donovan et al. (1997) and Liang and Stillman (1997). Spheroplasts prepared from nonsynchronized cells were lysed in a nonionic detergent. The insoluble nuclear fraction (P) was separated from the soluble proteins (S) by low-speed centrifugation and was resuspended in an equal volume as the supernatant. In some experiments, the pellet was further digested with DNase I and the solubilized chromatin fraction (Chr) was separated from the insoluble nuclear scaffold (Sc) by low speed centrifugation (see Materials and Methods). (B) Chromatin assays were performed on exponentially growing cultures of GA-850 (Dbf4–Myc) and GA-893 (Orc2–Myc). The antibodies used for Western blot analysis of total protein extracts (T), supernatant (S), and insoluble nuclear (P) fractions are described in Fig. 3 and in Materials and Methods. Mab414 (Berkeley Antibody Inc., CA) detects the nuclear pore proteins Nup100, Nup116 and Nup145, of which the central band (Nup116, indicated with bar) reproducibly partitions equally between soluble and insoluble nuclear fractions, providing an internal control for loading. In this panel the strain GA-893 (Orc2–Myc) was used for the detection of Orc2p. (C) Insoluble nuclear fraction from an exponentially growing culture of GA-850 cells (Dbf4–Myc) was further fractionated by DNase I digestion. Very little protein is recovered in the final scaffold pellet and therefore three times the amount was loaded to facilitate quantitation. Orc2p is detected with anti-Orc2p (JAb12, gift of J. Diffley), Mcm2p is detected with a goat polyclonal serum, YN-19, (Santa Cruz Antibodies, CA) and Dbf4–Myc with 9E10 on identical scaffold (Sc) and soluble chromatin (Chr) samples. Quantitation of protein distribution between the two fractions is shown on the graph at right. (D) Isogenic wild-type (GA-850) and orc2-1 (GA-1027) cells were arrested in G1 phase at 23°C with α-factor, were incubated at 37°C for 30 min in the presence of α-factor and were released from the block at 37°C for 60 min. After spheroplasting, permeabilization of the cells with Triton X-100 and centrifugation, the amount of Dbf4p in S and P fractions was quantitated by immunoblotting in three independent experiments. One-tenth of the supernatant was loaded on the gel relative to the pellet fraction. Nucleoporins (Nup) were used as loading control and for normalization. (E) FACS analysis of a typical experiment in which orc2-1 and wild-type cells were arrested with α-factor at permissive temperature (αF) and released for 60 min at 37°C (rel.).
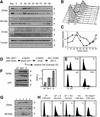
Figure 6
Dbf4p does not require Cdc6p or MCM loading to associate with the insoluble nuclear fraction. (A) GA-1213 cells (cdc15, GAL–CDC6) grown on galactose were arrested in late M phase for 2 hr at 37°C. Half of the culture was maintained for another hour in the same medium at 37°C whereas 2% glucose was added to the other half to shut off the GAL–CDC6 promoter. Cells were then released from the cdc15 arrest in the presence (GAL–CDC6 ON) or the absence (GAL–CDC6 OFF) of Cdc6 function. Samples were taken at regular time intervals and the DNA content of the cells was analyzed by FACS. (B) Chromatin assays were performed on the samples described above and the distribution of Dbf4p and Mcm2p in the soluble and insoluble nuclear fractions was analyzed by Western blot. The amount of Dbf4p and Mcm2p in the insoluble nuclear fraction was determined relative to Swi6, which was used as a loading control. The percentage of budded cells is shown for the same time points. (C) GA-1026 cells grown at 25°C were arrested for 1 hr with 20 μg/ml nocodazole and were shifted to 37°C for another hour in the presence of nocodazole before being released from the arrest at 37°C. Samples were taken just prior to (Noc) and at regular time intervals after the release. Position of cells in the cell cycle was determined by FACS and the distribution of Dbf4p and Mcm2p in the insoluble nuclear fraction, relative to p55RNase, was analyzed by Western blot as described in Materials and Methods. The quantitation represents the mean value for two experiments. Relative Dbf4p levels for wild-type and cdc6-1 Noc samples are 160 and 220, respectively.
Figure 7
The association of Dbf4p with chromatin is cell cycle regulated. (A) GA-850 cells arrested in G1 with α-factor were released into S phase, and aliquots were taken at regular time intervals. Cells were spheroplasted and fractionated into a soluble (S) and insoluble nuclear (P) fraction as described in Materials and Methods. The insoluble nuclear fraction was resuspended in 1/10 the volume of the supernatant. Equal amounts of proteins were loaded for each time point, and distribution of Dbf4p, Mcm2p, Orc2p, and tubulin was probed by Western blot. (B) FACS analysis of DNA content was performed on the cells described in A, as they synchronously traversed the cell cycle. (C) Quantitation of the relative amount of Dbf4p (○) and Mcm2p (●) in the insoluble nuclear fractions through the cell cycle, derived from the data shown in A. (D) GA-980 cells, that produce a nondegradable form of Sic1p in the presence of galactose, were grown on YPD and were arrested in G1 with α-factor for 90 min. Cells were then shifted to galactose-containing medium (YP–Gal) for 30 min in the presence of α-factor before being released from the pheromone arrest in YP–Gal for 2 hr. These cells block irreversibly at the G1/S transition with high Sic1p levels. In parallel, α-factor arrested GA-980 cells were grown on YPD and released into S phase until the appearance of small buds, indicative of entry into S phase. (E) FACS analysis of GA-980-arrested cells from D. (F) GA-980 cells arrested as described in D were spheroplasted and fractionated as described above (Fig. 5A) by the chromatin assay. The soluble (S, light gray bars) and insoluble nuclear (P, dark gray bars) fractions were analyzed by Western blot for Dbf4–Myc, and for p55RNase (Karwan et al. 1990). The distribution of Dbf4p was quantitated by immunoblotting and normalized to p55RNase, which shows no cell cycle variation. (G) GA-850 cells were arrested in G1 for 2 hr with α-factor (lane 1) and were released into S phase for 30 min in the presence (lane 3) or the absence (lane 2) of 200 μm of HU and the association of Dbf4p and Mcm2p with the insoluble nuclear fraction (P) was analyzed as described above. The same experiment was performed by arresting exponentially growing GA-850 cells (lane 4) and an isogenic strain deficient for the rad53 checkpoint function (GA-1212, lane 5) for 2.5 hr with 200 μm HU. (H) FACS analysis of samples described in G.
Figure 8
The chromatin-associated fraction of Dbf4p is displaced on initiation. (A) An in vitro DNA replication assay with G1 nuclei prepared from α-factor arrested GA-893 cells was performed to localize Orc2–Myc relative to the sites of incorporation of DIG–dUTP. The in vitro replication reaction with DIG–dUTP and its detection were performed as described in Figs. 1C and 4C. Bar, 2 μm. (B) A similar experiment with G1 nuclei (GA-850) shows that the Dbf4–Myc staining in replicating nuclei is inversely proportional to the amount of DIG–dUTP incorporated. The replication assay was stopped by addition of para-formaldehyde and both the sites of DIG–dUTP (green) incorporation and of Dbf4–Myc (red) localization were detected by IF. Different replicating nuclei presenting increasing levels of DIG–dUTP incorporation are shown. Bar, 2 μm. (C) An in vitro DNA replication assay was performed for 2 hr with GA-850 G1 nuclei, in the presence (+aph) or the absence (−aph) of aphidicolin. The nuclei were permeabilized with 1% Triton X-100 before (lanes labeled G1) or after the reaction and the presence of Dbf4p and Rpa2p was detected in the insoluble nuclear (P) and the soluble (S) fractions by immunoblotting. If replication proceeds (−aph), then most of Dbf4p is degraded by the end of the 2-hr incubation. If it is blocked (+aph), then the chromatin-associated Dbf4p remains. Because the ratio of insoluble nuclear to soluble Dbf4p is higher in isolated nuclei than in whole cell extracts, some soluble Dbf4p may be lost during nuclear isolation.
Figure 9
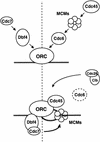
A scheme of two pathways that converge to create initiation-competent origins in yeast nuclei. The binding of MCMs and Cdc45p require the association of Cdc6p with ORC. Cdc6p and MCM binding is inhibited by high Cdk activity, whereas the binding of Cdc45p requires Cdk activity. We propose that the targeting of Cdc7p to the origin is mediated by Dbf4p in a manner independent of the activity state of Cdc28 kinase (the dashed line separates Cdk-independent steps from those regulated by Cdk-1). These two pathways converge, because the MCM proteins are physiological targets of Dbf4/Cdc7 and the two complexes interact (bottom; Lei et al. 1997). The activation of Clb5/Cdc28 as SPF, provokes the binding of Cdc45p, and the degradation or release of Cdc6p. Together the two kinase complexes promote initiation of DNA replication. The formation of initiation-competent origins thus depends on two unstable, factors that show cell cycle-regulated expression, Cdc6p and Dbf4p.
Similar articles
- Cdc7p-Dbf4p kinase binds to chromatin during S phase and is regulated by both the APC and the RAD53 checkpoint pathway.
Weinreich M, Stillman B. Weinreich M, et al. EMBO J. 1999 Oct 1;18(19):5334-46. doi: 10.1093/emboj/18.19.5334. EMBO J. 1999. PMID: 10508166 Free PMC article. - Association of RPA with chromosomal replication origins requires an Mcm protein, and is regulated by Rad53, and cyclin- and Dbf4-dependent kinases.
Tanaka T, Nasmyth K. Tanaka T, et al. EMBO J. 1998 Sep 1;17(17):5182-91. doi: 10.1093/emboj/17.17.5182. EMBO J. 1998. PMID: 9724654 Free PMC article. - A Cdc7p-Dbf4p protein kinase activity is conserved from yeast to humans.
Johnston LH, Masai H, Sugino A. Johnston LH, et al. Prog Cell Cycle Res. 2000;4:61-9. doi: 10.1007/978-1-4615-4253-7_6. Prog Cell Cycle Res. 2000. PMID: 10740815 Review. - First the CDKs, now the DDKs.
Johnston LH, Masai H, Sugino A. Johnston LH, et al. Trends Cell Biol. 1999 Jul;9(7):249-52. doi: 10.1016/s0962-8924(99)01586-x. Trends Cell Biol. 1999. PMID: 10370238 Review.
Cited by
- Highly stable loading of Mcm proteins onto chromatin in living cells requires replication to unload.
Kuipers MA, Stasevich TJ, Sasaki T, Wilson KA, Hazelwood KL, McNally JG, Davidson MW, Gilbert DM. Kuipers MA, et al. J Cell Biol. 2011 Jan 10;192(1):29-41. doi: 10.1083/jcb.201007111. J Cell Biol. 2011. PMID: 21220507 Free PMC article. - Cdc7-Dbf4 phosphorylates MCM proteins via a docking site-mediated mechanism to promote S phase progression.
Sheu YJ, Stillman B. Sheu YJ, et al. Mol Cell. 2006 Oct 6;24(1):101-13. doi: 10.1016/j.molcel.2006.07.033. Mol Cell. 2006. PMID: 17018296 Free PMC article. - Mms22p protects Saccharomyces cerevisiae from DNA damage induced by topoisomerase II.
Baldwin EL, Berger AC, Corbett AH, Osheroff N. Baldwin EL, et al. Nucleic Acids Res. 2005 Feb 17;33(3):1021-30. doi: 10.1093/nar/gki246. Print 2005. Nucleic Acids Res. 2005. PMID: 15718301 Free PMC article. - A Dual Inhibitor of Cdc7/Cdk9 Potently Suppresses T Cell Activation.
Chen EW, Tay NQ, Brzostek J, Gascoigne NRJ, Rybakin V. Chen EW, et al. Front Immunol. 2019 Jul 25;10:1718. doi: 10.3389/fimmu.2019.01718. eCollection 2019. Front Immunol. 2019. PMID: 31402912 Free PMC article. - An essential role for Orc6 in DNA replication through maintenance of pre-replicative complexes.
Semple JW, Da-Silva LF, Jervis EJ, Ah-Kee J, Al-Attar H, Kummer L, Heikkila JJ, Pasero P, Duncker BP. Semple JW, et al. EMBO J. 2006 Nov 1;25(21):5150-8. doi: 10.1038/sj.emboj.7601391. Epub 2006 Oct 19. EMBO J. 2006. PMID: 17053779 Free PMC article.
References
- Aparicio O M, Weinstein DM, Bell SP. Components and dynamics of DNA replication complexes in S. cerevisiae: Redistribution of MCM proteins and Cdc45p during S phase. Cell. 1997;91:59–69. - PubMed
- Bahman M, Buck V, White A, Rosamond J. Characterisation of the CDC7 gene product of Saccharomyces cerevisiae as a protein kinase needed for the initiation of mitotic DNA synthesis. Biochim Biophys Acta. 1988;951:335–343. - PubMed
- Blow JJ. Eukaryotic DNA replication (ed. J.J. Blow), pp. 143-165. IRL Press, Oxford, UK. 1996. Chromosome replication in Xenopus egg extracts.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases