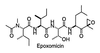Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity - PubMed (original) (raw)
Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity
L Meng et al. Proc Natl Acad Sci U S A. 1999.
Abstract
The proteasome regulates cellular processes as diverse as cell cycle progression and NF-kappaB activation. In this study, we show that the potent antitumor natural product epoxomicin specifically targets the proteasome. Utilizing biotinylated-epoxomicin as a molecular probe, we demonstrate that epoxomicin covalently binds to the LMP7, X, MECL1, and Z catalytic subunits of the proteasome. Enzymatic analyses with purified bovine erythrocyte proteasome reveal that epoxomicin potently inhibits primarily the chymotrypsin-like activity. The trypsin-like and peptidyl-glutamyl peptide hydrolyzing catalytic activities also are inhibited at 100- and 1,000-fold slower rates, respectively. In contrast to peptide aldehyde proteasome inhibitors, epoxomicin does not inhibit nonproteasomal proteases such trypsin, chymotrypsin, papain, calpain, and cathepsin B at concentrations of up to 50 microM. In addition, epoxomicin is a more potent inhibitor of the chymotrypsin-like activity than lactacystin and the peptide vinyl sulfone NLVS. Epoxomicin also effectively inhibits NF-kappaB activation in vitro and potently blocks in vivo inflammation in the murine ear edema assay. These results thus define epoxomicin as a novel proteasome inhibitor that likely will prove useful in exploring the role of the proteasome in various in vivo and in vitro systems.
Figures
Figure 1
Structure of epoxomicin.
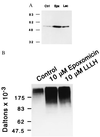
Figure 2
Epoxomicin biotin binds four proteasome catalytic subunits. EL4 murine thymoma cells (3 × 105) were incubated with 5 μM epoxomicin-biotin without (lane 1) and with (lane 2) 50 μM epoxomicin pretreatment. Human B cell lymphoma cells LCL 721.45 (lane 3) and LCL 721.174 (lane 4) were labeled with 5 μM epoxomicin-biotin. Biotinylated proteins were visualized by using avidin-horseradish peroxidase and chemiluminescence.
Figure 3
Accumulation of p53 (A) and ubiquitinated proteins (B) in epoxomicin-treated cells. (A) α-p53 immunoblot analyses of HUVECs treated with 100 nM epoxomicin (Epx), 5 μM lactacystin (Lac), or vehicle (ctrl) for 6 hr. (B) α-Ubiquitin immunoblot analyses of HeLa cells treated with 10 μM epoxomicin or peptide inhibitor Z-LLL-H for 2 hr.
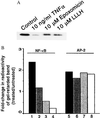
Figure 4
Epoxomicin inhibits activation of NF-κB. (A) IκBα degradation induced by TNF-α is prevented by epoxomicin. HeLa cells were treated with epoxomicin (10 μM) or Z-LLL-H (10 μM) for 2 hr and subsequently treated with TNF-α (10 ng/ml) for 15 min. Western blot analysis of cell lysates was performed to measure IκBα levels as in Fig. 3. (B) EMSA analysis of NF-κB DNA-binding activity. HeLa cells were treated with increasing concentrations of epoxomicin for 2 hr, and, subsequently, 10 ng/ml TNF-α was added to drug-treated cells or to untreated cultures and incubated for 1 hr. Equal amounts of protein from nuclear extracts prepared from untreated and treated cultures were incubated with a radiolabeled NF-κB oligonucleotide or a control AP-2 oligonucleotide and fractionated on 4% polyacrylamide gels. Dried gels were exposed to a PhosphorImaging screen. The amount of radioactivity in the transcription factor-retarded bands was quantitated and represented as fold-change of treated over that of untreated samples. Shown are TNF-α alone (lanes 1 and 5) and TNF-α plus epoxomicin (100 nM, lanes 2 and 6; 1 μM, lanes 3 and 7; and 10 μM, lanes 4 and 8).
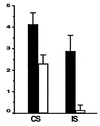
Figure 5
Epoxomicin inhibits inflammation in vivo. (A) CS to picrylchloride. BALB/c mice were immunized with picrylchloride by topical application to shaved chest and abdomen. One group of immunized mice (n = 4) was treated with epoxomicin daily from the time of immunization until the time of ear challenge, whereas the control immunized group (n = 4) was treated with vehicle. On day 6 postimmunization, all mice were skin-challenged by painting both ears with 0.8% picrylchloride. Ear swelling responses were measured before (0 hr) and 24 hr after ear challenge. (B) Irritant sensitivity (IS) response to picrylchloride. Nonimmunized BALB/c mice were injected with epoxomicin (n = 4) or vehicle (n = 4) before ear challenge with 0.8% picrylchloride, and ear swelling measurements were made 0 and 24 hr post-ear challenge. Results in CS and IS assays are expressed as 24-hr measurement minus 0-hr measurement.
Similar articles
- Total synthesis of the potent proteasome inhibitor epoxomicin: a useful tool for understanding proteasome biology.
Sin N, Kim KB, Elofsson M, Meng L, Auth H, Kwok BH, Crews CM. Sin N, et al. Bioorg Med Chem Lett. 1999 Aug 2;9(15):2283-8. doi: 10.1016/s0960-894x(99)00376-5. Bioorg Med Chem Lett. 1999. PMID: 10465562 - Towards subunit-specific proteasome inhibitors: synthesis and evaluation of peptide alpha',beta'-epoxyketones.
Elofsson M, Splittgerber U, Myung J, Mohan R, Crews CM. Elofsson M, et al. Chem Biol. 1999 Nov;6(11):811-22. doi: 10.1016/s1074-5521(99)80128-8. Chem Biol. 1999. PMID: 10574782 - The selective proteasome inhibitors lactacystin and epoxomicin can be used to either up- or down-regulate antigen presentation at nontoxic doses.
Schwarz K, de Giuli R, Schmidtke G, Kostka S, van den Broek M, Kim KB, Crews CM, Kraft R, Groettrup M. Schwarz K, et al. J Immunol. 2000 Jun 15;164(12):6147-57. doi: 10.4049/jimmunol.164.12.6147. J Immunol. 2000. PMID: 10843664 Free PMC article. - Proteasome inhibitors and antigen presentation.
Bogyo M, Gaczynska M, Ploegh HL. Bogyo M, et al. Biopolymers. 1997;43(4):269-80. doi: 10.1002/(SICI)1097-0282(1997)43:4<269::AID-BIP2>3.0.CO;2-T. Biopolymers. 1997. PMID: 9316392 Review. - Catalytic activities of the 20 S proteasome, a multicatalytic proteinase complex.
Orlowski M, Wilk S. Orlowski M, et al. Arch Biochem Biophys. 2000 Nov 1;383(1):1-16. doi: 10.1006/abbi.2000.2036. Arch Biochem Biophys. 2000. PMID: 11097171 Review.
Cited by
- Identification of novel host-targeted compounds that protect from anthrax lethal toxin-induced cell death.
Slater LH, Hett EC, Mark K, Chumbler NM, Patel D, Lacy DB, Collier RJ, Hung DT. Slater LH, et al. ACS Chem Biol. 2013 Apr 19;8(4):812-22. doi: 10.1021/cb300555n. Epub 2013 Feb 4. ACS Chem Biol. 2013. PMID: 23343607 Free PMC article. - The Role of Calpain and Proteasomes in the Degradation of Carbonylated Neuronal Cytoskeletal Proteins in Acute Experimental Autoimmune Encephalomyelitis.
Smerjac SM, Zheng J, Hu CL, Bizzozero OA. Smerjac SM, et al. Neurochem Res. 2018 Dec;43(12):2277-2287. doi: 10.1007/s11064-018-2648-y. Epub 2018 Sep 24. Neurochem Res. 2018. PMID: 30251207 - Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma.
Kuhn DJ, Chen Q, Voorhees PM, Strader JS, Shenk KD, Sun CM, Demo SD, Bennett MK, van Leeuwen FW, Chanan-Khan AA, Orlowski RZ. Kuhn DJ, et al. Blood. 2007 Nov 1;110(9):3281-90. doi: 10.1182/blood-2007-01-065888. Epub 2007 Jun 25. Blood. 2007. PMID: 17591945 Free PMC article. - The ubiquitin-proteasome system.
Nandi D, Tahiliani P, Kumar A, Chandu D. Nandi D, et al. J Biosci. 2006 Mar;31(1):137-55. doi: 10.1007/BF02705243. J Biosci. 2006. PMID: 16595883 Review. - Binding of LARP6 to the conserved 5' stem-loop regulates translation of mRNAs encoding type I collagen.
Cai L, Fritz D, Stefanovic L, Stefanovic B. Cai L, et al. J Mol Biol. 2010 Jan 15;395(2):309-26. doi: 10.1016/j.jmb.2009.11.020. Epub 2009 Nov 13. J Mol Biol. 2010. PMID: 19917293 Free PMC article.
References
- Varshavsky A. Trends Biochem Sci. 1997;22:383–387. - PubMed
- Hilt W, Wolf D H. Trends Biochem Sci. 1996;21:96–102. - PubMed
- Groll M, Ditzel L, Lowe J, Stock D, Bochtler M, Bartunik H D, Huber R. Nature (London) 1997;386:463–471. - PubMed
- Driscoll J, Brown M G, Finley D, Monaco J J. Nature (London) 1993;365:262–264. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous