Dynamic visualization of nervous system in live Drosophila - PubMed (original) (raw)
Dynamic visualization of nervous system in live Drosophila
B Sun et al. Proc Natl Acad Sci U S A. 1999.
Abstract
We have constructed transgenic Drosophila melanogaster lines that express green fluorescent protein (GFP) exclusively in the nervous system. Expression is controlled with transcriptional regulatory elements present in the 5' flanking DNA of the Drosophila Na(+), K(+)-ATPase beta-subunit gene Nervana2 (Nrv2). This regulatory DNA is fused to the yeast transcriptional activator GAL4, which binds specifically to a sequence motif termed the UAS (upstream activating sequence). Drosophila lines carrying Nrv2-GAL4 transgenes have been genetically recombined with UAS-GFP (S65T) transgenes (Nrv2-GAL4+UAS-GFP) inserted on the same chromosomes. We observe strong nervous system-specific fluorescence in embryos, larvae, pupae, and adults. The GFP fluorescence is sufficiently bright to allow dynamic imaging of the nervous system at all of these developmental stages directly through the cuticle of live Drosophila. These lines provide an unprecedented view of the nervous system in living animals and will be valuable tools for investigating a number of developmental, physiological, and genetic neurobiological problems.
Figures
Figure 1
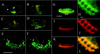
GFP fluorescence and double-labeled antibody staining in embryos. A live stage-12 embryo (A) shows green fluorescent neurons in the anterior end (arrowhead) and in the ventral nerve cord (asterisk). The ventral nerve cord neurons are partially obscured by the strong yellow autofluorescence of the gut at this stage. In stage-15 embryos (B), some of the peripheral sensory neurons near the surface as well as many cells in the ventral nerve cord are visible. By stage 16 (C) and 17 (D) more cells in the ventral nerve cord and brain and a few peripheral sensory neurons show fluorescence. The same field of view is shown for a stage-17 embryo in E near the surface and F more internally. All embryos are oriented with anterior to the left and ventral to the bottom. A_–_D are color charge-coupled device images, E and F are false-colored black and white charge-coupled device images. Fixed, stage-12 embryos were double stained with anti-GFP (G and H) and anti-HRP (I and J) antibodies. The hazy green area in G is yellow autofluorescence of the gut. The complete nervous system is stained with anti-GFP antibody (G) and is shown at higher power (H) or is stained with anti-HRP antibody (I) and is shown at higher power (J). Images in G and I as well as H and J were merged to produce K and L, respectively. There is a complete correspondence of the anti-GFP and anti-HRP staining. Note also the difference between GFP fluorescence in a live stage-12 embryo (A) and anti-GFP antibody staining of a similarly aged fixed embryo (G and H). The images in G_–_L are false-colored. br, brain; gt, developing gut; vnc, ventral nerve cord. (Bar = 100 μm for whole embryos, 10 μm for higher power views in H, J, and L.)
Figure 2
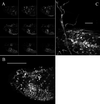
Confocal microscopy. (A) The nine small images are individual optical sections (spaced 6 μm apart) taken from the anterior of a stage-17 embryo. (B) Twenty-eight optical sections from the same embryo (spaced 2 μm apart) are projected to reconstruct the complete three-dimensional distribution of fluorescence. The single and double arrows indicate especially bright peripheral neurons. An animation of this projection series is available as supplemental data on the PNAS web site (
). (C) A higher power view of fluorescence in the central part of the ventral nerve cord of an early first-instar larvae. Twenty optical sections (spaced 2.5 μm apart) are projected to reconstruct the three-dimensional view. The fluorescence is clearly present in most central nervous system cells and processes. All images are oriented with anterior to the left and ventral down. [Bar = 100 μm (B) and 10 μm (C).]
Figure 3
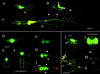
GFP fluorescence in larvae, pupae, and adults. First- (A), second- (B), and third- (C) instar larvae show intense fluorescence in the brain and ventral ganglion as well as the longitudinal and segmental nerves and nerve branches. The large bright structures at the ends of segmental nerves may be peripheral glia (indicated by arrowheads). The specimens in A and C are viewed from the ventral surface whereas B is a lateral view. The image in C has been constructed from images of three smaller fields. In early pupae (D), the fluorescence is similar to late third-instar larvae. As metamorphosis proceeds, the nerves begin to appear wavy and frayed (E, and at higher power in F). Later stages of pupation show decreased fluorescence (G) and a shape more characteristic of adult central nervous system. Leg nerves are visible in late-stage pupae (H) or a few hours before hatching (I) and are indicated with arrowheads. Whole adults show bright fluorescence in the optic lobes, brain, thoracic ganglion, and many kinds of nerves. In J, the thoracic ganglion, leg, and probosal nerves are evident, while the optic lobe and brain staining is obscured by the red eye. An adult female abdomen shows major nerves with many finer branches at higher power (K). A dissected head viewed from the posterior shows the intense fluorescence in the brain, optic lobes, and proboscal nerves (L). The leg nerve (M) and wing nerve (N) are also clearly visible in dissected parts from adults. an, abdominal nerve; br, brain; ey, eye; ln, longitudinal nerve; ol, optic lobe; pb, proboscis; sn, segmental nerve; tg, thoracic ganglion; vnc, ventral nerve cord. (Bars = 100 μm.)
Similar articles
- Organization and transcriptional regulation of Drosophila Na(+), K(+)-ATPase beta subunit genes: Nrv1 and Nrv2.
Xu P, Sun B, Salvaterra PM. Xu P, et al. Gene. 1999 Aug 20;236(2):303-13. doi: 10.1016/s0378-1119(99)00269-3. Gene. 1999. PMID: 10452950 - Green fluorescent protein/beta-galactosidase double reporters for visualizing Drosophila gene expression patterns.
Timmons L, Becker J, Barthmaier P, Fyrberg C, Shearn A, Fyrberg E. Timmons L, et al. Dev Genet. 1997;20(4):338-47. doi: 10.1002/(SICI)1520-6408(1997)20:4<338::AID-DVG5>3.0.CO;2-8. Dev Genet. 1997. PMID: 9254908 - Green fluorescent protein as a vital marker and reporter of gene expression in Drosophila.
Yeh E, Gustafson K, Boulianne GL. Yeh E, et al. Proc Natl Acad Sci U S A. 1995 Jul 18;92(15):7036-40. doi: 10.1073/pnas.92.15.7036. Proc Natl Acad Sci U S A. 1995. PMID: 7624365 Free PMC article. - deGradFP: A System to Knockdown GFP-Tagged Proteins.
Caussinus E, Affolter M. Caussinus E, et al. Methods Mol Biol. 2016;1478:177-187. doi: 10.1007/978-1-4939-6371-3_9. Methods Mol Biol. 2016. PMID: 27730581 Review. - Ectopic gene expression in Drosophila using GAL4 system.
Phelps CB, Brand AH. Phelps CB, et al. Methods. 1998 Apr;14(4):367-79. doi: 10.1006/meth.1998.0592. Methods. 1998. PMID: 9608508 Review.
Cited by
- A putative amino acid transporter of the solute carrier 6 family is upregulated by lithium and is required for resistance to lithium toxicity in Drosophila.
Kasuya J, Kaas GA, Kitamoto T. Kasuya J, et al. Neuroscience. 2009 Oct 20;163(3):825-37. doi: 10.1016/j.neuroscience.2009.07.027. Epub 2009 Jul 18. Neuroscience. 2009. PMID: 19619614 Free PMC article. - Predetermined embryonic glial cells form the distinct glial sheaths of the Drosophila peripheral nervous system.
von Hilchen CM, Bustos AE, Giangrande A, Technau GM, Altenhein B. von Hilchen CM, et al. Development. 2013 Sep;140(17):3657-68. doi: 10.1242/dev.093245. Epub 2013 Jul 31. Development. 2013. PMID: 23903191 Free PMC article. - A genome-wide resource for the analysis of protein localisation in Drosophila.
Sarov M, Barz C, Jambor H, Hein MY, Schmied C, Suchold D, Stender B, Janosch S, K J VV, Krishnan RT, Krishnamoorthy A, Ferreira IR, Ejsmont RK, Finkl K, Hasse S, Kämpfer P, Plewka N, Vinis E, Schloissnig S, Knust E, Hartenstein V, Mann M, Ramaswami M, VijayRaghavan K, Tomancak P, Schnorrer F. Sarov M, et al. Elife. 2016 Feb 20;5:e12068. doi: 10.7554/eLife.12068. Elife. 2016. PMID: 26896675 Free PMC article. - Glial investment of the adult and developing antennal lobe of Drosophila.
Oland LA, Biebelhausen JP, Tolbert LP. Oland LA, et al. J Comp Neurol. 2008 Aug 10;509(5):526-50. doi: 10.1002/cne.21762. J Comp Neurol. 2008. PMID: 18537134 Free PMC article. - Lithium chloride alleviates neurodegeneration partly by inhibiting activity of GSK3β in a SCA3 Drosophila model.
Jia DD, Zhang L, Chen Z, Wang CR, Huang FZ, Duan RH, Xia K, Tang BS, Jiang H. Jia DD, et al. Cerebellum. 2013 Dec;12(6):892-901. doi: 10.1007/s12311-013-0498-3. Cerebellum. 2013. PMID: 23812869
References
- Chalfie M, Tu Y, Euskirchen G, Ward W W, Prasher D C. Science. 1994;263:802–805. - PubMed
- Prasher D C. Trends Genet. 1995;11:320–323. - PubMed
- Gerdes H H, Kaether C. FEBS Lett. 1996;389:44–47. - PubMed
- Brand A. Trends Genet. 1995;11:324–325. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases