An African green monkey lacking peripheral CD4 lymphocytes that retains helper T cell activity and coexists with SIVagm - PubMed (original) (raw)
An African green monkey lacking peripheral CD4 lymphocytes that retains helper T cell activity and coexists with SIVagm
Y Murayama et al. Clin Exp Immunol. 1999 Sep.
Abstract
Natural infection with simian immunodeficiency virus (SIV) is known to occur in the African green monkey (AGM). The actual onset of the disease has not been recognized in SIVagm infected AGM, and the precise reason for such apathogenicity in the AGM remains unclear. We reported previously that AGM peripheral CD4 lymphocytes underwent a peculiar differentiation from CD4+ to CD4- cells after in vitro activation, and we inferred that the AGM does not fall into a fatal immunodeficient state because of the generation of CD4- helper T cells in vivo. To evaluate this possibility, we examined the relationship between CD4 expression and helper T cell activity in the naturally infected AGM. We identified a healthy monkey almost lacking CD4 T cells in the periphery. This AGM showed no signs and symptoms of immunodeficiency and retained a helper T cell activity in antibody production comparable to those of CD4+ AGMs. In addition, SIVagm could be isolated from CD8+ lymphocytes in the CD4- AGM. These observations suggest that a unique host-virus adaptation has developed in the AGM, and may be helpful in explaining the fundamental reason for the apathogenicity occurring in this monkey.
Figures
Fig. 1
(a) CD4 and CD8 expressions in simian immunodeficiency virus (SIV) seronegative (Agm−) and seropositive (Agm1–3) monkey peripheral blood lymphocytes (PBL). Two-colour flow cytometric analysis was performed with forward-and right-angle scatter gates set on the lymphocyte fraction. 104 cells were counted. Negative control cells were stained with control phycoerythrin- (PE) and fluorescein-isothiocyanate- (FITC) immunoglobulin (Ig) G antibodies. The sorting gates were set on the rectangles as indicated. (b) CD4 gene expression in peripheral blood mononuclear cells (PBMC). The total RNA was isolated from PBMC and the expressions of mRNA were analysed by the reverse transcriptase-polymerase chain reaction. (c) pokeweed mitogen (PWM)-induced antibody production in the African green monkey (AGM). PBMC were cultured in medium with (closed bars) or without (open bars) PWM for 1 week. In Agm1–3, the inhibitory effect for PWM-induced antibody synthesis by CD4 antibody was also examined (grey bars). The IgG released into the culture supernatant was estimated by an indirect enzyme-linked immunosorbent assay. (d) Proliferative response of Agm3 PBMC against SIV-uninfected (open bar) and SIV-infected (closed bar) CemX174 cells. The cell proliferation was estimated by AlamarBlue assay and the response of control PBMC incubated with SIV-negative CemX174 cells was represented as 100%.
Fig. 2
(a) Detection of proviral DNA in CD4 and CD8 subsets in Agm3. DNA was extracted from sorted 102–104 cells and employed for nested-polymerase chain reaction (PCR). (b) Detection of SIVagm provirus by the PCR and Southern blotting in Cemx174 cells cocultured with peripheral CD4 or CD8 cells from African green monkeys. A negative reaction was confirmed in the DNA sample of CemX174 cells cultured alone. (c) Multiple alignment of deduced amino acid sequences of SIV isolated from vervet monkeys. PCR derived env nucleotide sequences from Agm2 CD4 cells (verCD4) and Agm3 CD8 cells (verCD8) were translated and aligned with previously reported SIV env sequences. Dashes indicate sequence identity with the consensus sequence, while dots represent gaps introduced to optimize the alignment. ‘2’ in the consensus sequence denotes sites in which two amino acids were shared in viruses at the same frequency. Asterisks indicate cysteine residues. V3, V4 and V5 designate variable regions. The putative CD4 binding site and HIV-1 V3 loop equivalent were as described previously [16,26]. The sequences of the references were obtained from the DNA data bank of Japan (DDBJ): ZA40 (AF015906), ZA358 (AF015905), IPR806 (AF015907), IPR859 (AF015908) and IPR1185 (AF015809) [30]; ver1 (U04003) and ver2 (U04004) [31]; ver155 (M29975) [32]; VER266 (U10896) and VER385 (U10898) [33]; 9063 (L40990) [34]; ver3 (M30931) [35]; and TYO1 (X07805) [1]. 9063 was isolated from a pig-tailed macaque inoculated with SIVagm90.
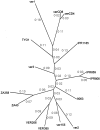
Fig. 3
Unrooted phylogenetic tree of SIVagm isolated from vervet monkeys. Phylogenetic relationships were estimated by the maximum likelihood method. The values in the figure indicate the rate of divergence.
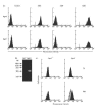
Fig. 4
(a) CD3, CD4 and CD8 expressions in cultured lymphocytes. Peripheral blood mononuclear cells from Agm1 and Agm− were cultured with interleukin-2 and ConA for 30 days and examined by flow cytometry. Negative control cells were stained with control antibodies. (b) Detection of SIVagm provirus by polymerase chain reaction and Southern blotting in cultured lymphocytes. (c) Intracellular expression of gag protein in cultured lymphocytes stained with the anti-HIV gag monoclonal antibody, VAK-4. Negative control cells were incubated with mouse immunoglobulin G. The data for the control (grey histograms) and the sample stained with VAK-4 antibody (black histograms) can be seen to overlap on the figure. The VAK-4 positive percentages at 7 days (upper row) and 30 days (lower row) of cultivation in Agm1 were about 7% and 53%, respectively.
Similar articles
- CD8+ T lymphocytes of African green monkeys secrete an immunodeficiency virus-suppressing lymphokine.
Ennen J, Findeklee H, Dittmar MT, Norley S, Ernst M, Kurth R. Ennen J, et al. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7207-11. doi: 10.1073/pnas.91.15.7207. Proc Natl Acad Sci U S A. 1994. PMID: 7913749 Free PMC article. - CD4 and CD8 expressions in African green monkey helper T lymphocytes: implication for resistance to SIV infection.
Murayama Y, Amano A, Mukai R, Shibata H, Matsunaga S, Takahashi H, Yoshikawa Y, Hayami M, Noguchi A. Murayama Y, et al. Int Immunol. 1997 Jun;9(6):843-51. doi: 10.1093/intimm/9.6.843. Int Immunol. 1997. PMID: 9199967 - Simian immunodeficiency virus SIVagm.sab infection of Caribbean African green monkeys: a new model for the study of SIV pathogenesis in natural hosts.
Pandrea I, Apetrei C, Dufour J, Dillon N, Barbercheck J, Metzger M, Jacquelin B, Bohm R, Marx PA, Barre-Sinoussi F, Hirsch VM, Müller-Trutwin MC, Lackner AA, Veazey RS. Pandrea I, et al. J Virol. 2006 May;80(10):4858-67. doi: 10.1128/JVI.80.10.4858-4867.2006. J Virol. 2006. PMID: 16641277 Free PMC article. - SIVagm infection of its natural African green monkey host.
Norley SG. Norley SG. Immunol Lett. 1996 Jun;51(1-2):53-8. doi: 10.1016/0165-2478(96)02555-2. Immunol Lett. 1996. PMID: 8811345 Review. - SIVagm: genetic and biological features associated with replication.
Müller MC, Barré-Sinoussi F. Müller MC, et al. Front Biosci. 2003 Sep 1;8:d1170-85. doi: 10.2741/1130. Front Biosci. 2003. PMID: 12957815 Review.
Cited by
- Epigenetic silencing of CD4 expression in nonpathogenic SIV infection in African green monkeys.
Mudd JC, Lai S, Shah S, Rahmberg A, Flynn JK, Starke CE, Perkins MR, Ransier A, Darko S, Douek DC, Hirsch VM, Cameron M, Brenchley JM. Mudd JC, et al. JCI Insight. 2020 Sep 17;5(18):e139043. doi: 10.1172/jci.insight.139043. JCI Insight. 2020. PMID: 32841214 Free PMC article. - Loss of memory CD4+ T-cells in semi-wild mandrills (Mandrillus sphinx) naturally infected with species-specific simian immunodeficiency virus SIVmnd-1.
Greenwood EJD, Schmidt F, Liégeois F, Kondova I, Herbert A, Ngoubangoye B, Rouet F, Heeney JL. Greenwood EJD, et al. J Gen Virol. 2014 Jan;95(Pt 1):201-212. doi: 10.1099/vir.0.059808-0. Epub 2013 Nov 8. J Gen Virol. 2014. PMID: 24214347 Free PMC article. - Memory CD4(+) T lymphocytes in the gastrointestinal tract are a major source of cell-associated simian immunodeficiency virus in chronic nonpathogenic infection of African green monkeys.
Schmitz JE, Ma ZM, Hagan EA, Wilks AB, Furr KL, Linde CH, Zahn RC, Brenchley JM, Miller CJ, Permar SR. Schmitz JE, et al. J Virol. 2012 Oct;86(20):11380-5. doi: 10.1128/JVI.01556-12. Epub 2012 Aug 15. J Virol. 2012. PMID: 22896600 Free PMC article. - Interleukin-2 Therapy Induces CD4 Downregulation, Which Decreases Circulating CD4 T Cell Counts, in African Green Monkeys.
Mudd JC, Perkins MR, DiNapoli SR, Hirsch VM, Brenchley JM. Mudd JC, et al. J Virol. 2016 May 27;90(12):5750-5758. doi: 10.1128/JVI.00057-16. Print 2016 Jun 15. J Virol. 2016. PMID: 27053558 Free PMC article. - Multivariate profiling of African green monkey and rhesus macaque T lymphocytes.
Hassan WM, Burton GF, Pinter GA, Lauko IG, Mahdi NN, Johnson ME. Hassan WM, et al. Sci Rep. 2019 Mar 18;9(1):4834. doi: 10.1038/s41598-019-41209-x. Sci Rep. 2019. PMID: 30886198 Free PMC article.
References
- Fukasawa M, Miura T, Hasegawa A, et al. Sequence of simian immunodeficiency virus from African green monkey, a new member of the HIV/SIV group. Nature. 1988;333:457–61. - PubMed
- Tsujimoto H, Hasegawa A, Maki N, et al. Sequence of a novel simian immunodeficiency virus from a wild-caught African mandrill. Nature. 1989;341:539–41. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials