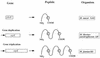Bioenergetics of the Archaea - PubMed (original) (raw)
Review
Bioenergetics of the Archaea
G Schäfer et al. Microbiol Mol Biol Rev. 1999 Sep.
Abstract
In the late 1970s, on the basis of rRNA phylogeny, Archaea (archaebacteria) was identified as a distinct domain of life besides Bacteria (eubacteria) and Eucarya. Though forming a separate domain, Archaea display an enormous diversity of lifestyles and metabolic capabilities. Many archaeal species are adapted to extreme environments with respect to salinity, temperatures around the boiling point of water, and/or extremely alkaline or acidic pH. This has posed the challenge of studying the molecular and mechanistic bases on which these organisms can cope with such adverse conditions. This review considers our cumulative knowledge on archaeal mechanisms of primary energy conservation, in relationship to those of bacteria and eucarya. Although the universal principle of chemiosmotic energy conservation also holds for Archaea, distinct features have been discovered with respect to novel ion-transducing, membrane-residing protein complexes and the use of novel cofactors in bioenergetics of methanogenesis. From aerobically respiring Archaea, unusual electron-transporting supercomplexes could be isolated and functionally resolved, and a proposal on the organization of archaeal electron transport chains has been presented. The unique functions of archaeal rhodopsins as sensory systems and as proton or chloride pumps have been elucidated on the basis of recent structural information on the atomic scale. Whereas components of methanogenesis and of phototrophic energy transduction in halobacteria appear to be unique to Archaea, respiratory complexes and the ATP synthase exhibit some chimeric features with respect to their evolutionary origin. Nevertheless, archaeal ATP synthases are to be considered distinct members of this family of secondary energy transducers. A major challenge to future investigations is the development of archaeal genetic transformation systems, in order to gain access to the regulation of bioenergetic systems and to overproducers of archaeal membrane proteins as a prerequisite for their crystallization.
Figures
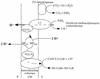
FIG. 1
Phylogenetic tree. The scheme demonstrates the division into Crenarchaeota and Euryarchaeota and shows the position of the major archaeal genera. The tree was redrawn according to references , , and . Stars denote archaeal species for which specific bioenergetic information has been found.
FIG. 2
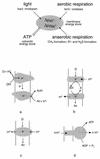
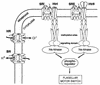
Primary energy-transducing processes and coupling principles in membrane bioenergetics. The top scheme illustrates the processes found in archaea that contribute to the formation of either proton or sodium ion potentials across the plasma membrane. Details are discussed throughout this review. The bottom schemes illustrate three mechanisms by which an ion gradient can be produced: (a) chemical charge separation (only electrons are transferred through the membrane); (b) a mobile membrane-integral cofactor like the quinones or methanophenazine functioning as proton transporter (examples are _bc_1 complexes); and (c) redox-driven pumps like cyt c oxidase. All schemes are drawn for an H+/e− ratio of 1. Scheme d illustrates the proton-driven ATP synthase of the FoF1 or A1Ao type as an example for a secondary energy transducer. D, electron donor; Ac, electron acceptor.
FIG. 3
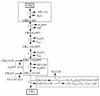
Pathways of methanogenesis. Reactions involved in energy conservation are boxed. The reduction of methyl-CoM (reactions 6 and 7) is common to all methanogenic substrates. During methane formation from H2 plus CO2, reactions 1 to 5 proceed in the direction of CO2 reduction. The methyl groups of methanol and acetate enter the central pathway at the level of H4MPT. During methanogenesis from methanol, one-fourth of the methanol is oxidized to CO2 by the reversal of reactions 1 to 5; the six reducing equivalents gained are used to reduce 3 mol of methanol to methane. During methanogenesis from acetate, the carboxyl group is oxidized to CO2 and the electrons gained are used to reduce the methyl group to acetate. F420, oxidized form of coenzyme F420; F420H2, reduced form of F420; HS-CoM, CoM (2-mercaptoethanesulfonate); HS-CoB, CoB (7-mercaptoheptanoylthreonine phosphate); CoM-S-S-CoB, heterodisulfide of HS-CoM and HS-CoB. Enzymes: 1, formyl-MF dehydrogenase; 2, formyl-MF:H4MPT formyltransferase and methenyl-H4MPT cyclohydrolase; 3, F420-dependent methylene-H4MPT dehydrogenase; 4, F420-dependent methylene-H4MPT reductase; 5, methyl-H4MPT:CoM-methyltransferase; 6, methyl-CoM reductase; 7, heterodisulfide reductase system (different electron donor systems are indicated).
FIG. 4
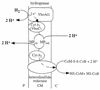
Tentative scheme of electron flow and proton translocation during heterodisulfide reduction with H2 as electron donor. This reaction sequence is part of methanogenesis from H2-CO2. This scheme is valid for methylotrophic methanogens only, for hydrogenotrophic methanogens do not contain cytochromes and the presence of methanophenazine (MP) has not been verified. The heterodisulfide reductase is not indicated to be a proton pump, but this cannot be ruled out a priori. This scheme is based on the experimentally derived stoichiometry of 3 to 4 H+ translocated/methyl group reduced. P, periplasm; CM, cytoplasmic membrane; C, cytoplasm. For explanations, see the text.
FIG. 5
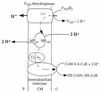
Tentative scheme of electron flow and proton translocation during heterodisulfide reduction with F420H2 as electron donor. This reaction sequence is part of methanogenesis from methanol, methylamines, and formate. This scheme is valid for methylotrophic methanogens only (see the legend to Fig. 4). F420, coenzyme F420; MP, methanophenazine; P, periplasm; CM, cytoplasmic membrane; C, cytoplasm. For explanations, see the text.
FIG. 6
Tentative scheme of electron flow and proton translocation coupled to heterodisulfide reduction with CO as electron donor. This reaction sequence is part of methanogenesis from acetate. The presence of methanophenazine (MP) in acetate-grown cells has not been verified. Fd, ferredoxin; P, periplasm; CM, cytoplasmic membrane; C, cytoplasm. For explanations, see the text.
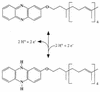
FIG. 7
Structure and reactivity of methanophenazine, a membrane-integral electron and hydrogen carrier of methanogens.
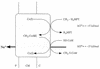
FIG. 8
Tentative scheme of the reaction mechanism of the Na+-translocating methyl-H4MPT:CoM-methyltransferase. The enzyme is a multisubunit enzyme consisting of eight nonidentical subunits in unknown stoichiometry. The reaction can be divided into two partial reactions, methylation and demethylation of an enzyme-bound corrinoid cofactor. The demethylation reaction is apparently coupled to Na+ transport. Co(I) and Co(III) denote different valence states of the enzyme-bound corrinoid cofactor. HS-CoM, CoM (2-mercaptoethanesulfonate); P, periplasm; CM, cytoplasmic membrane; C, cytoplasm. For explanations, see the text.
FIG. 9
Gene organization within archaeal SDH operons. Numbers indicate the calculated molecular masses (in kilodaltons) of the gene products. In all cases, subunit A is the flavin-containing dehydrogenase polypeptide; subunit B refers to the iron-sulfur protein in complex II or fumarate reductase, respectively. The columns on the right indicate the type of quinone used as terminal electron acceptor in vivo and the number of heme B molecules present in the complex. MQ, menaquinone; TQ, Thermoplasma quinone, CQ, caldariella quinone. tmh, putative transmembrane helices. E.coli, E. coli (256); B.sub., B. subtilis (353); T.ac., T. acidophilum (29); S.ac., S. acidocaldarius (254) (accession no. Y09041); N.ph., N. pharaonis (accession no. Y07709). The assignment of the open reading frames was done by analyzing sequence similarities. From Thermoplasma, only a partially sequenced operon has been reported; ORF-1 has homology to sdhB and frdB; ORF-2 is a putative diheme cyt b.
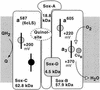
FIG. 10
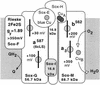
Composition of the SoxABCD terminal oxidase of S. acidocaldarius. The shaded areas represent the membrane. Each box represents a single constituent polypeptide with its type of redox center and the redox potential measured at pH 6.5. The dashed oval insert illustrates the proposed location of the binding site for caldariella quinone at an interface between subunit II (SoxA) and the cyt b analog SoxC. Further details are given in the text.
FIG. 11
Composition of the respiratory supercomplex SoxM. Each box of the scheme represents a constituent polypeptide together with its redox centers and the respective redox potentials (where known). The location of the blue copper protein sulfocyanine is hypothetical, as a possible link between the partial complexes of SoxG-SoxF and SoxM-SoxH. For details, see the text.
FIG. 12
Gene organization of archaeal terminal oxidases. The operon structure is compared to those of E. coli and P. denitrificans. Numbers within the boxes refer to the molecular masses (in kilodaltons) of the gene products; arrows indicate the direction of transcription. The assignment of genes to subunits of the oxidase complexes is given below the boxes; the darkly shaded box in all cases signifies subunit I, identifying the complex as a member of the heme-Cu oxidase family. In the case of A. ambivalens, the two operons are located far apart on the genome, whereas the soxM locus of S. acidocaldarius forms a close gene cluster. Data were compiled from references , , , , , , , , and and/or the EMBL data bank. su, subunit.
FIG. 13
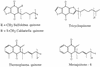
Structures of respiratory quinones isolated from membranes of thermoacidophilic and halophilic archaea.
FIG. 14
Structures of hemes found in archaeal terminal oxidases. The hemes are assigned according to references and .
FIG. 15
Schematic representation of the electron transport chains of S. acidocaldarius and A. ambivalens. The brackets encompass components coded for by a single gene cluster or operon. Brackets with dashed lines signify functional units which may operate as proton pumps. The position of sulfocyanine in the upper scheme is hypothetical at present. The presence of CuA is inferred from the binding motif in subunit II of SoxM. The question mark in the A. ambivalens chain underscores the lack of information concerning the path of electrons from sulfur oxidation to caldariella quinone.
FIG. 16
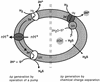
Possible mechanisms for ΔμH+ generation by sulfur reduction. (Left) H2 oxidation and S0 reduction both occur on the outside. (Right) The redox reactions take place on opposite sides with respect to the membrane. For details, see the text. Hy, hydrogenase; SR, sulfur reductase.
FIG. 17
Amino acid residues of subunit I involved in proton pumping by terminal oxidases. The boxed area encloses the archaeal terminal oxidases which are compared to the bacterial ones from T. thermophilus and P. denitrificans; the latter serves as a reference enzyme with known three-dimensional structure. Column 1 identifies the positions of the critical amino acids within individual transmembrane helices or the helix II-III connecting loop; + and − denote the presence and absence, respectively, of a specific residue in the enzymes; letters and numbers in parentheses describe positions and types of the replacement amino acids in the archaeal organisms. All oxidases which lack the motif in loop II-III are considered to be incapable of pumping protons by the mechanism employed by cyt c oxidase. Details and conclusions from this analysis are described in the text. “a,” numbering according to P. denitrificans; “b” refers to entire motif (-Nx10Dx6N-). P.den., P. denitrificans; H.sal., H. salinarum; S.ac., S. acidocaldarius; A.amb., A. ambivalens; N.pha., N. pharonis; T.th., T. thermophilus.
FIG. 18
Structural model of BR (A) and proton transfer steps (B). (A) Structural model of BR adapted from reference . The retinal chromophore is depicted as dark gray circles. The charged residues on the cytoplasmic side may function as proton collectors that funnel a proton to residue Asp-96. The EC channel comprises several charged amino acids, including Asp-212, Asp-85, and Arg-82. (B) Correlation of proton transfer steps with the spectroscopically determined transitions of intermediates. The two proton transfers occurring during the L ⇒ M transition (≈10 μs) depict the deprotonation of the Schiff base concomitant with the protonation of Asp-85 and the ejection of a proton to the extracellular side. In the subsequent steps, a proton from Asp-96 reprotonates the Schiff base (M ⇒ N). Asp-96 receives a proton from the cytoplasm during the N ⇒ O transition. Finally, the proton reservoir in the CP channel (H+ in the dotted circle) is replenished by the protonated Asp-85, which completes the cycle. The thermal reisomerization of 13-cis retinal to all-trans retinal occurs during the N ⇒ O transition. The protein switch that separates the accessibility between the CP and EC channels is thought to occur between two different M intermediates.
FIG. 19
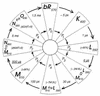
Scheme of the BR photocycle based on a sequential irreversible model according to reference . P0 to P7 denote the spectroscopically defined intermediates. The four states P3, P4, P7, and P6 are quasiequilibria between M and L, M and N, and N and O. The decay rates (at 20°C) of the intermediates are depicted between the wings.
FIG. 20
The four rhodopsins of H. salinarum. The two ion pumps, BR and HR, convert light energy into a proton and a chloride gradient, respectively, which are utilized by the cell for its energy-requiring steps, e.g., ATP synthesis, metabolism, and/or ion transport. The two other retinylidene pigments, SRI and SRII, function as photoreceptors, which direct the bacteria to optimal light conditions. The external stimuli, light and chemoeffectors, activate their corresponding receptors, i.e., the SRs, SRI and SRII, or the chemoreceptors. The SRs form complexes with their homodimeric transducers, HtrI and HtrII. After the absorption of light, the signal is transferred to the cytoplasmic domain of the transducers, which consists of three parts. Directly following the two membrane-spanning helices are small domains that are specific for the Htrs. The methylation sites downstream are involved in processes of adaptation to constant stimuli. Finally, the signaling domain interacts with a His kinase (CheA), which then transfers this information via the phosphoregulator (CheY) to the flagellar motor switch. (The figure is reproduced from reference with the kind permission of J. L. Spudich.)
FIG. 21
Structure of A1Ao ATPase-encoding genes in archaea. Genes encoding similar polypeptides are indicated by the same pattern. Genes encoding hydrophobic polypeptides are marked by asterisks. It is suggested but not supported by experimental data that MT0952 and MJ0614 are related to ATPase function or assembly. For gene-polypeptide correspondence, see the text.
FIG. 22
Gene-polypeptide correspondence in proteolipids from methanoarchaea. The proteolipid-encoding gene of M. mazei (atpK) codes for an 8-kDa polypeptide with two transmembrane helices connected by a loop. It is suggested to fold in the membrane like a hairpin. Helix 2 contains the proton-translocating residue. The proteolipid-encoding genes (atpK) of M. thermoautotrophicum and M. jannaschii arose by duplication and triplication, respectively, of an ancestral gene with subsequent fusion of the genes. The proteolipids from M. thermoautotrophicum and M. jannaschii are predicted to have two and three predicted hairpins, respectively. The duplicated proteolipid from M. thermoautotrophicum contains the proton-translocating residue in both hairpins, but the triplicated form of M. jannaschii lacks it in the first of three hairpins.
FIG. 23
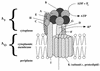
Hypothetical structure of the A1Ao ATPase from M. mazei. The model is based on experimental data and by analogy to F1Fo and V1Vo ATPases. It is not clear whether AhaG and AhaH are indeed part of the structure. The localization of AhaC, AhaD, AhaE, AhaF, AhaG, and AhaH is speculative. Note that this model does not apply to all methanoarchaeal ATPases, for the number of proteolipid monomers is likely to differ, depending on the size of the monomer. Furthermore, no homologs of AhaG can be identified with certainty in other archaea.
Similar articles
- On the origin of respiration: electron transport proteins from archaea to man.
Schäfer G, Purschke W, Schmidt CL. Schäfer G, et al. FEMS Microbiol Rev. 1996 May;18(2-3):173-88. doi: 10.1111/j.1574-6976.1996.tb00235.x. FEMS Microbiol Rev. 1996. PMID: 8639327 Review. - ATP synthases from archaea: the beauty of a molecular motor.
Grüber G, Manimekalai MS, Mayer F, Müller V. Grüber G, et al. Biochim Biophys Acta. 2014 Jun;1837(6):940-52. doi: 10.1016/j.bbabio.2014.03.004. Epub 2014 Mar 17. Biochim Biophys Acta. 2014. PMID: 24650628 Review. - Life close to the thermodynamic limit: how methanogenic archaea conserve energy.
Deppenmeier U, Müller V. Deppenmeier U, et al. Results Probl Cell Differ. 2008;45:123-52. doi: 10.1007/400_2006_026. Results Probl Cell Differ. 2008. PMID: 17713742 Review. - A bioenergetic basis for membrane divergence in archaea and bacteria.
Sojo V, Pomiankowski A, Lane N. Sojo V, et al. PLoS Biol. 2014 Aug 12;12(8):e1001926. doi: 10.1371/journal.pbio.1001926. eCollection 2014 Aug. PLoS Biol. 2014. PMID: 25116890 Free PMC article. - Respiratory chains of archaea and extremophiles.
Schäfer G, Purschke WG, Gleissner M, Schmidt CL. Schäfer G, et al. Biochim Biophys Acta. 1996 Jul 18;1275(1-2):16-20. doi: 10.1016/0005-2728(96)00043-6. Biochim Biophys Acta. 1996. PMID: 8688447 Review.
Cited by
- Cytochromes c in Archaea: distribution, maturation, cell architecture, and the special case of Ignicoccus hospitalis.
Kletzin A, Heimerl T, Flechsler J, van Niftrik L, Rachel R, Klingl A. Kletzin A, et al. Front Microbiol. 2015 May 12;6:439. doi: 10.3389/fmicb.2015.00439. eCollection 2015. Front Microbiol. 2015. PMID: 26029183 Free PMC article. - ATP synthase: Evolution, energetics, and membrane interactions.
Nirody JA, Budin I, Rangamani P. Nirody JA, et al. J Gen Physiol. 2020 Nov 2;152(11):e201912475. doi: 10.1085/jgp.201912475. J Gen Physiol. 2020. PMID: 32966553 Free PMC article. Review. - Halorubrum pleomorphic virus-6 Membrane Fusion Is Triggered by an S-Layer Component of Its Haloarchaeal Host.
Bignon EA, Chou KR, Roine E, Tischler ND. Bignon EA, et al. Viruses. 2022 Jan 27;14(2):254. doi: 10.3390/v14020254. Viruses. 2022. PMID: 35215847 Free PMC article. - Minimization of extracellular space as a driving force in prokaryote association and the origin of eukaryotes.
Hooper SL, Burstein HJ. Hooper SL, et al. Biol Direct. 2014 Nov 18;9(1):24. doi: 10.1186/1745-6150-9-24. Biol Direct. 2014. PMID: 25406691 Free PMC article. - 'Whole Organism', Systems Biology, and Top-Down Criteria for Evaluating Scenarios for the Origin of Life.
Brunk CF, Marshall CR. Brunk CF, et al. Life (Basel). 2021 Jul 14;11(7):690. doi: 10.3390/life11070690. Life (Basel). 2021. PMID: 34357062 Free PMC article. Review.
References
- Abken H J, Bäumer S, Brodersen J, Murakami E, Ragsdale S W, Gottschalk G, Deppenmeier U. Membrane-bound electron transport and H+ translocation in Methanosarcina barkeri Gö1. Biospectrum, special issue, March. 1998. p. 38.
- Abken H J, Deppenmeier U. Purification and properties of an F420H2 dehydrogenase from Methanosarcina mazei Gö1. FEMS Microbiol Lett. 1997;154:231–237.
- Abrahams J P, Leslie A G W, Lutter R, Walker J E. Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature. 1994;370:621–628. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases