Studies in B7-deficient mice reveal a critical role for B7 costimulation in both induction and effector phases of experimental autoimmune encephalomyelitis - PubMed (original) (raw)
Studies in B7-deficient mice reveal a critical role for B7 costimulation in both induction and effector phases of experimental autoimmune encephalomyelitis
T T Chang et al. J Exp Med. 1999.
Abstract
The importance of B7 costimulation in regulating T cell expansion and peripheral tolerance suggests that it may also play a significant regulatory role in the development of autoimmune disease. It is unclear whether B7 costimulation is involved only in the expansion of autoreactive T cells in the periphery, or if it is also required for effector activation of autoreactive T cells in the target organ for mediating tissue injury and propagating autoimmune disease. In this study, the role of B7-CD28 costimulation and the relative importance of B7 costimulators for the induction and effector phases of experimental autoimmune encephalomyelitis (EAE) induced by myelin oligodendrocyte glycoprotein (MOG) peptide were examined. Wild-type, B7-1/B7-2-deficient mice, or CD28-deficient C57BL/6 mice were immunized with MOG 35-55 peptide. Mice lacking both B7-1 and B7-2 or CD28 showed no or minimal clinical signs of EAE and markedly reduced inflammatory infiltrates in the brain and spinal cord. However, mice lacking either B7-1 or B7-2 alone developed clinical and pathologic EAE that was comparable to EAE in wild-type mice, indicating overlapping functions for B7-1 and B7-2. Resistance to EAE was not due to a lack of induction of T helper type 1 (Th1) cytokines, since T cells from B7-1/B7-2(-/-) mice show reduced proliferative responses, but greater interferon gamma production compared with T cells from wild-type mice. To study the role of B7 molecules in the effector phase of the disease, MOG 35-55-specific T lines were adoptively transferred into the B7-1/B7-2(-/-) and wild-type mice. Clinical and histologic EAE were markedly reduced in B7-1/B7-2(-/-) compared with wild-type recipient mice. These results demonstrate that B7 costimulation has critical roles not only in the initial activation and expansion of MOG-reactive T cells, but also in the effector phase of encephalitogenic T cell activation within the central nervous system.
Figures
Figure 1
EAE disease course in C57BL/6 B7-1−/− mice (A; n = 34), B7-2−/− mice (B; n = 22), B7-1/B7-2−/− mice (C; n = 18), and CD28−/− mice (D; n = 13). Filled diamonds in A–D, wild-type (Wt) mice. Mice were immunized with 100 μg of MOG 35-55 subcutaneously and injected twice with 200 ng of pertussis toxin intravenously. Mice were observed daily and scored as described in Materials and Methods. Data are pooled from three to five experiments and represent mean clinical score of each group plotted against time.
Figure 2
CNS histopathology. Representative fields from the anterior lumbar spinal cord of wild-type (A), B7-2−/− (B), and B7-1/B7-2−/− (C) mice from day 35 after immunization. In A and B, there are many mononuclear inflammatory cells in the leptomeninges and infiltrating the parenchyma. In C, there is a small meningeal inflammatory focus (arrow), but the parenchyma is not infiltrated. All are stained with Luxol fast blue–hematoxylin and eosin. Original magnifications: 280×.
Figure 3
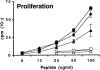
Proliferation and cytokine production of lymph node cells from B7-1−/− and B7-2−/− mice challenged with MOG 35-55. Mice were immunized with 100 μg of MOG 35-55 in CFA. 10 d later, draining lymph node cells from wild-type (Wt, diamonds), B7-1−/− (squares), or B7-2−/− (triangles) mice were restimulated in vitro with a titration of MOG 35-55 (filled symbols) or MOG 92-106 (open symbols) as control. Culture supernatants were collected after 48 h from cultures stimulated with 50 μg/ml of MOG 35-55 or with media alone. Cytokines were measured by sandwich ELISA, and background levels from media controls were subtracted. Data are representative of eight independent experiments.
Figure 3
Proliferation and cytokine production of lymph node cells from B7-1−/− and B7-2−/− mice challenged with MOG 35-55. Mice were immunized with 100 μg of MOG 35-55 in CFA. 10 d later, draining lymph node cells from wild-type (Wt, diamonds), B7-1−/− (squares), or B7-2−/− (triangles) mice were restimulated in vitro with a titration of MOG 35-55 (filled symbols) or MOG 92-106 (open symbols) as control. Culture supernatants were collected after 48 h from cultures stimulated with 50 μg/ml of MOG 35-55 or with media alone. Cytokines were measured by sandwich ELISA, and background levels from media controls were subtracted. Data are representative of eight independent experiments.
Figure 4
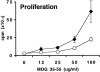
Proliferation and cytokine production of lymph node cells from B7-1/B7-2−/− mice challenged with MOG 35-55. Mice were immunized with 100 μg of MOG 35-55 in CFA. 10 d later, draining lymph node cells from wild-type (diamonds) or B7-1/B7-2−/− (circles) mice were restimulated in vitro with a range of MOG 35-55 peptide concentrations. Supernatants were collected after 24, 48, and 72 h from cultures stimulated with 50 μg/ml of MOG 35-55 or with media alone. Cytokines from wild-type (Wt, solid bars) and B7-1/B7-2−/− (hatched bars) cultures were measured by sandwich ELISA, and background levels from media controls were subtracted. Data are representative of eight independent experiments.
Figure 4
Proliferation and cytokine production of lymph node cells from B7-1/B7-2−/− mice challenged with MOG 35-55. Mice were immunized with 100 μg of MOG 35-55 in CFA. 10 d later, draining lymph node cells from wild-type (diamonds) or B7-1/B7-2−/− (circles) mice were restimulated in vitro with a range of MOG 35-55 peptide concentrations. Supernatants were collected after 24, 48, and 72 h from cultures stimulated with 50 μg/ml of MOG 35-55 or with media alone. Cytokines from wild-type (Wt, solid bars) and B7-1/B7-2−/− (hatched bars) cultures were measured by sandwich ELISA, and background levels from media controls were subtracted. Data are representative of eight independent experiments.
Figure 5
EAE induction in wild-type and B7-1/B7-2−/− mice by adoptive transfer of wild-type T cells. Wild-type mice were immunized with MOG 35-55, and draining lymph nodes were harvested 10 d later. Lymphocytes were cultured in MOG 35-55 and IL-12 for 4 d and then transferred into wild-type (Wt, n = 16) and B7.1/B7.2−/− (n = 13) recipients. Data are pooled from two experiments and represent mean clinical score plotted against time. B7-1/B7-2−/− mice developed significantly less severe disease compared with wild-type. *P < 0.05 by the Mann-Whitney test.
Similar articles
- A critical role for B7/CD28 costimulation in experimental autoimmune encephalomyelitis: a comparative study using costimulatory molecule-deficient mice and monoclonal antibody blockade.
Girvin AM, Dal Canto MC, Rhee L, Salomon B, Sharpe A, Bluestone JA, Miller SD. Girvin AM, et al. J Immunol. 2000 Jan 1;164(1):136-43. doi: 10.4049/jimmunol.164.1.136. J Immunol. 2000. PMID: 10605004 - CD28-independent induction of experimental autoimmune encephalomyelitis.
Chitnis T, Najafian N, Abdallah KA, Dong V, Yagita H, Sayegh MH, Khoury SJ. Chitnis T, et al. J Clin Invest. 2001 Mar;107(5):575-83. doi: 10.1172/JCI11220. J Clin Invest. 2001. PMID: 11238558 Free PMC article. - De novo central nervous system processing of myelin antigen is required for the initiation of experimental autoimmune encephalomyelitis.
Tompkins SM, Padilla J, Dal Canto MC, Ting JP, Van Kaer L, Miller SD. Tompkins SM, et al. J Immunol. 2002 Apr 15;168(8):4173-83. doi: 10.4049/jimmunol.168.8.4173. J Immunol. 2002. PMID: 11937578 - Differential requirements of naïve and memory T cells for CD28 costimulation in autoimmune pathogenesis.
Perrin PJ, Lovett-Racke A, Phillips SM, Racke MK. Perrin PJ, et al. Histol Histopathol. 1999 Oct;14(4):1269-76. doi: 10.14670/HH-14.1269. Histol Histopathol. 1999. PMID: 10506942 Review. - The role of costimulation in autoimmune demyelination.
Racke MK, Ratts RB, Arredondo L, Perrin PJ, Lovett-Racke A. Racke MK, et al. J Neuroimmunol. 2000 Jul 24;107(2):205-15. doi: 10.1016/s0165-5728(00)00230-7. J Neuroimmunol. 2000. PMID: 10854658 Review.
Cited by
- Regulation and Function of the PD-L1 Checkpoint.
Sun C, Mezzadra R, Schumacher TN. Sun C, et al. Immunity. 2018 Mar 20;48(3):434-452. doi: 10.1016/j.immuni.2018.03.014. Immunity. 2018. PMID: 29562194 Free PMC article. Review. - CD86 is expressed on murine hematopoietic stem cells and denotes lymphopoietic potential.
Shimazu T, Iida R, Zhang Q, Welner RS, Medina KL, Alberola-Lla J, Kincade PW. Shimazu T, et al. Blood. 2012 May 24;119(21):4889-97. doi: 10.1182/blood-2011-10-388736. Epub 2012 Feb 27. Blood. 2012. PMID: 22371880 Free PMC article. - Spontaneous autoimmunity in the absence of IL-2 is driven by uncontrolled dendritic cells.
Isakson SH, Katzman SD, Hoyer KK. Isakson SH, et al. J Immunol. 2012 Aug 15;189(4):1585-93. doi: 10.4049/jimmunol.1200342. Epub 2012 Jul 9. J Immunol. 2012. PMID: 22778392 Free PMC article. - Targeted knock-in mice expressing mutations of CD28 reveal an essential pathway for costimulation.
Dodson LF, Boomer JS, Deppong CM, Shah DD, Sim J, Bricker TL, Russell JH, Green JM. Dodson LF, et al. Mol Cell Biol. 2009 Jul;29(13):3710-21. doi: 10.1128/MCB.01869-08. Epub 2009 Apr 27. Mol Cell Biol. 2009. PMID: 19398586 Free PMC article.
References
- McAdam A.J., Schweitzer A.N., Sharpe A.H. The role of B7 co-stimulation in activation and differentiation of CD4+ and CD8+ T cells. Immunol. Rev. 1998;165:231–247. - PubMed
- Kuchroo V., Das M.P., Brown J.A., Ranger A.M., Zamvil S.S., Sobel R.A., Weiner H.L., Nabavi N., Glimcher L.H. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathwaysapplication to autoimmune disease therapy. Cell. 1995;80:707–718. - PubMed
- Miller S.D., Vanderlugt C.L., Lenschow D.J., Pope J.G., Karandikar N.J., Dal Canto M.C., Bluestone J.A. Blockade of CD28/B7-1 interaction prevents epitope spreading and clinical relapses of murine EAE. Immunity. 1995;3:739–745. - PubMed
- Borriello F., Sethna M.P., Boyd S.D., Schweitzer A.N., Tivol E.A., Jacoby D., Strom T.B., Simpson E.M., Freeman G.J., Sharpe A.H. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS35685/NS/NINDS NIH HHS/United States
- NS26773/NS/NINDS NIH HHS/United States
- P01 AI039671/AI/NIAID NIH HHS/United States
- R01 NS035685/NS/NINDS NIH HHS/United States
- P01 AI39671/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases