Two types of K(+) channel subunit, Erg1 and KCNQ2/3, contribute to the M-like current in a mammalian neuronal cell - PubMed (original) (raw)
Two types of K(+) channel subunit, Erg1 and KCNQ2/3, contribute to the M-like current in a mammalian neuronal cell
A A Selyanko et al. J Neurosci. 1999.
Abstract
The potassium M current was originally identified in sympathetic ganglion cells, and analogous currents have been reported in some central neurons and also in some neural cell lines. It has recently been suggested that the M channel in sympathetic neurons comprises a heteromultimer of KCNQ2 and KCNQ3 (Wang et al., 1998) but it is unclear whether all other M-like currents are generated by these channels. Here we report that the M-like current previously described in NG108-15 mouse neuroblastoma x rat glioma cells has two components, "fast" and "slow", that may be differentiated kinetically and pharmacologically. We provide evidence from PCR analysis and expression studies to indicate that these two components are mediated by two distinct molecular species of K(+) channel: the fast component resembles that in sympathetic ganglia and is probably carried by KCNQ2/3 channels, whereas the slow component appears to be carried by merg1a channels. Thus, the channels generating M-like currents in different cells may be heterogeneous in molecular composition.
Figures
Fig. 1.
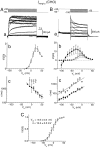
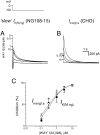
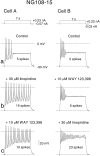
Pharmacological separation of two components of the M-like (_I_K(M,ng)) current in NG108–15 mouse neuroblastoma x rat glioma cells. M-like currents recorded in two cells (A, B) as deactivating tail currents produced by voltage steps from −20 mV (holding potential) to −50 mV. In each cell, the control current had two different components, fast and slow, which could be blocked by WAY 123,398 and linopirdine, respectively.Insets on the right show the difference (blocker-sensitive) currents. The current in A consisted predominantly of the slow component (blocked by WAY 123,398), whereas that in B was predominantly fast and blocked by linopirdine.
Fig. 2.
Kinetic analysis of the M-like current in a NG108–15 mouse neuroblastoma x rat glioma cell. Total_I_K(M,ng) was activated by holding at −20 mV and then deactivated by a 6 sec step (top record) to −50 mV before (A) and after (B) addition of the _erg_-channel blocker WAY 123,398 (10 μ
m
). In control (A) the deactivation tail was fitted (smooth line, superimposed) by the sum of fast (τ = 76 msec, 254 pA) and two slow (τ = 340 msec, 37 pA and 2124 msec, 70 pA) exponential curves. WAY 123,398 abolished both slow components without affecting the fast component (B, τ = 76 msec). C, Slow component obtained by subtracting the record shown in B from that shown in_A_ was fitted (smooth line, superimposed) by the sum of two kinetic components (τ = 315 and 1972 msec). All records were obtained from the same cell. Dashed lines denote zero current levels.
Fig. 3.
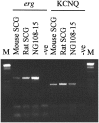
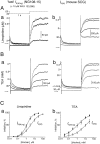
cDNA from mouse SCG, rat SCG, and chemically differentiated NG108–15 cells, was amplified using primers to the_erg_ family of potassium channel genes or primers recognizing KCNQ2 and KCNQ3 potassium channel genes. Amplified products were obtained from all cell types but not from a negative control containing no template, indicating that members of these families are expressed by these cells. Sequence analysis revealed that the amplified product obtained from NG108–15 cDNA with primers to the erg genes was predominantly, if not exclusively, merg1. Analysis of the amplified product obtained using the KCNQ primers in both rat SCG and NG108–15 has shown these cells express both KCNQ2 and_KCNQ3_. M-1 kb ladder DNA size standards.
Fig. 4.
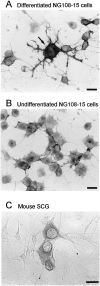
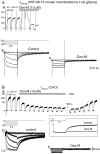
Immunocytochemical detection of merg1 protein in differentiated NG108 cells. Immunostaining for the C terminus of merg1 in chemically differentiated (A) and undifferentiated (B) NG108 cells, and in dissociated mouse SCG cells (C). Note that in_A_, a large NG108–15 cell (arrow) showed labeling of strong intensity, whereas adjacent smaller and bipolar cells were not stained. Micrographs were obtained using bright-field optics. Scale bars, 20 μm.
Fig. 5.
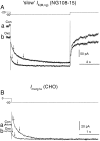
Characteristics of the merg1a current (I_merg1a) expressed in CHO cells.I_merg1a was activated by long (8 sec) depolarizing voltage steps from the holding level of −80 mV (Aa) and deactivated by hyperpolarizing steps after its full activation by a prepulse to +50 mV (Ba). Leak-subtracted steady-state I–V relationships obtained at the end of the depolarizing and hyperpolarizing pulses, respectively, are shown in Ab and Bb(open circles), and an “instantaneous”_I–V relationship obtained at the beginning of the hyperpolarizing pulses for current deactivation is shown in_Bb (filled circles). Activation (Ac) and deactivation (Bc) time constants were plotted semilogarithmically, and τ–V_relationships were fitted by straight lines with τ at 0 mV and the slope equal to 1218 ± 1 msec and −0.013 ± 0.001 mV−1 in Ac and 403 ± 1 msec and 0.012 ± 0.0006 mV−1 (filled circles) and 3437 ± 1 msec and 0.014 ± 0.002 mV−1 (open circles) in_Bc. C, Activation curve fitted by the Boltzmann equation at V_1/2 = −5.9 ± 0.6 mV and k = 12.2 ± 0.5 mV. Records in_Aa and Ba were from the same cell. In_Ab, Ac_, Bb, Bc, and C the mean data are shown (vertical lines indicate SEMs) obtained from six and nine cells, respectively.
Fig. 6.
Comparison of the slow M-like (_I_K(M,ng)) current in NG108–15 mouse neuroblastoma x rat glioma cells (A) and mouse-erg1a current (_I_merg1a) expressed in CHO cells (B). The slowly deactivating component of _I_K(M,ng)(Aa) was recorded in response to long hyperpolarizing steps (holding level, −20 mV; step duration, 6 sec; interval, 60 sec; increment, −10 mV). These slow _I_K(M,ng)currents were obtained by subtracting the currents recorded in the presence of 10 μ
m
WAY 123,398 (box, right) from those in the absence of the blocker (box, left). For comparison (Ba), I_merg1ais shown, obtained with a similar voltage protocol (note the holding potential of 0 mV in Ba). Deactivation tails were fitted by double-exponential curves (smooth lines, superimposed). Leak-subtracted steady-state (filled circles) and instantaneous (open circles)I–V relationships for_I_K(M,ng) (Ab) and_I_merg1a (Ba) were obtained by measuring the current at the beginning and end of the voltage pulse. Fast (filled circles) and slow (open circles) time constants for deactivation of_I_K(M,ng) (Ac) and_I_merg1a (Bc) were plotted semilogarithmically against membrane potential, and τ–_V relationships were fitted by straight lines with τ at 0 mV and the slope equal to 2134 ± 1 msec and 0.015 ± 0.0009 mV−1 (filled circles) and 15737 ± 1 msec and 0.016 ± 0.002 mV−1 (open circles) in_Ac_ and 680 ± 1 msec and 0.011 ± 0.0008 mV−1 (filled circles) and 5432 ± 1 msec and 0.012 ± 0.0007 mV−1(open circles) in Bc. In Ab, Ac, Bb, and Bc the mean data are shown (vertical lines indicate SEMs) obtained from 26 and 29 cells, respectively.
Fig. 7.
The slow component of the NG108–15 M-like current and the merg1a current are equally sensitive to WAY 123,398. Records in_A_ and B show the slow component of_I_K(M,ng) and_I_merg1a deactivation tail currents recorded on stepping from 0 to −50 mV in the presence of increasing concentrations of WAY 123,398 (0, 0.3, 1, and 10 μ
m
). Plots in C show mean percent inhibition of the tail currents (open circles,_I_K(M,ng); n = 4;filled circles, _I_merg1a; n = 6). See Table 2 for fitted parameters.
Fig. 8.
Characteristics of the fast M-like (_I_K(M,ng)) current in a NG108–15 mouse neuroblastoma x rat glioma cell (A) and the M current (_I_K(M)) in a mouse SCG neuron (B). The fast-deactivating component of_I_K(M,ng) (Aa) was recorded in the presence of 10 μ
m
WAY 123,398 at different membrane potentials (holding level, −20 mV; step duration, 1 sec; interval, 10 sec; increment, −10 mV). Box, Inhibition of the slow, WAY-sensitive component in this cell: currents before and after application of 10 μ
m
WAY 123,398, left, and the difference current, right. For comparison (Ba), _I_K(M) is shown, obtained with the same voltage protocol. Deactivation tails were fitted by single exponential curves (smooth lines, superimposed). Leak-subtracted steady-state (filled circles) and instantaneous (open circles)I–V relationships for_I_K(M,ng) (Ab) and_I_K(M) (Ba) were obtained by measuring the current at the beginning and end of the voltage pulse. When the time constants for deactivation of_I_K(M,ng) (Ac) and_I_K(M) (Bc) were plotted semilogarithmically against membrane potential, τ–_V_relationships were fitted by straight lines with τ at 0 mV and the slope equal to 271 ± 1 msec and 0.009 ± 0.0004 mV−1 in Ac and 399 ± 1 msec and 0.013 ± 0.0009 mV−1 in Bc. In Ab, Ac, Bb, and Bc the mean data are shown (vertical lines indicate SEM) obtained from 30 and 15 cells, respectively.
Fig. 9.
Differential sensitivities of fast_I_K(M,ng) and_I_K(M) to linopirdine and TEA. The fast (WAY-insensitive) component of _I_K(M,ng) and_I_K(M) was recorded in response to 1 sec steps from the holding level of −20 to −50 mV, in the absence and presence of different concentrations of linopirdine (Aa, Ab) and TEA (Ba, Bb).C, Concentration dependences of inhibition of the two currents by linopirdine (a) and TEA (b). Smooth lines are the fits by the Hill equation. For the parameters of the fit see Table 2.
Fig. 10.
Muscarinic inhibition of slow_I_K(M,ng) in a NG108–15 mouse neuroblastoma x rat glioma cell (A) and_I_merg1a expressed in a CHO cell (B). Currents (Aa, Ba) were produced by holding at −20 mV (A) or 0 mV (B) and giving repeated steps (at 0.02 Hz in_A_ and 0.025 Hz in B) to −50 mV for 6 sec. Both steady-state currents at the holding potentials and deactivation currents at the test potential were reduced by bath-application of oxotremorine-M (Oxo-M; 10 μ
m
). In an NG108–15 cell, inhibition of_I_K(M,ng) was preceded by a transient activation of a Ca2+-activated K+current (Aa). Families of currents in b_and c were obtained in response to incremental (−10 mV) hyperpolarizing voltage steps before (b) and during (c) action of Oxo-M. The_insert in B shows superimposed current produced by voltage steps from 0 to −50 mV before and during the action of Oxo-M.
Fig. 11.
Muscarinic inhibition of slow_I_K(M,ng) in a NG108–15 mouse neuroblastoma x rat glioma cell (A) and_I_merg1a expressed in a CHO cell (B) is accompanied by acceleration of their deactivation kinetics. Superimposed are deactivation tails obtained by stepping to −50 mV from −20 mV (A) or 0 mV (B) in control (Con) and in the presence of 10 μ
m
oxotremorine-M (Oxo).Smooth lines are double-exponential fits with time constants (indicated by arrows) equal to 276 and 1271 msec (Con) and 176 and 708 msec (Oxo) in_A_ and 92 and 398 msec (Con) and 71 and 290 msec (Oxo) in B.
Fig. 12.
Muscarinic inhibition of fast_I_K(M,ng) in an NG108–15 mouse neuroblastoma x rat glioma cell (A) and_I_K(M) in a mouse sympathetic neuron (B). Fast-deactivating_I_K(M,ng) currents (A) are shown during 1 sec of hyperpolarization from −20 to −50 mV, before and during the action of oxotremorine-M (Oxo-M; 10 μ
m
). Both currents were obtained in the presence of 10 μ
m
WAY 123,398. (The effect of WAY 123,398 on the total I_K(M,ng)recorded in this cell with a longer, 6 sec pulse, is shown in the_box.) Currents in a mouse sympathetic neuron were obtained before and after addition of WAY 123,398 and WAY 123,398 + Oxo-M.
Fig. 13.
Effects of inhibiting fast and slow_I_K(M,ng) on firing in NG108–15 cells. Records show action potential trains in two NG108–15 cells (A, B) produced by long (7 sec) depolarizing pulses (top records) from the holding potential of −90 mV in the absence of drugs (Aa, Ba), in the presence of 30 μ
m
linopirdine (Ab), 10 μ
m
WAY 123,398 (Bb), or in the presence of both linopirdine and WAY 123,398 (Ac, Bc).
Similar articles
- Activation of expressed KCNQ potassium currents and native neuronal M-type potassium currents by the anti-convulsant drug retigabine.
Tatulian L, Delmas P, Abogadie FC, Brown DA. Tatulian L, et al. J Neurosci. 2001 Aug 1;21(15):5535-45. doi: 10.1523/JNEUROSCI.21-15-05535.2001. J Neurosci. 2001. PMID: 11466425 Free PMC article. - Separation of M-like current and ERG current in NG108-15 cells.
Meves H, Schwarz JR, Wulfsen I. Meves H, et al. Br J Pharmacol. 1999 Jul;127(5):1213-23. doi: 10.1038/sj.bjp.0702642. Br J Pharmacol. 1999. PMID: 10455268 Free PMC article. - Dominant-negative subunits reveal potassium channel families that contribute to M-like potassium currents.
Selyanko AA, Delmas P, Hadley JK, Tatulian L, Wood IC, Mistry M, London B, Brown DA. Selyanko AA, et al. J Neurosci. 2002 Mar 1;22(5):RC212. doi: 10.1523/JNEUROSCI.22-05-j0001.2002. J Neurosci. 2002. PMID: 11880533 Free PMC article. - Both linopirdine- and WAY123,398-sensitive components of I K(M,ng) are modulated by cyclic ADP ribose in NG108-15 cells.
Higashida H, Brown DA, Robbins J. Higashida H, et al. Pflugers Arch. 2000 Dec;441(2-3):228-34. doi: 10.1007/s004240000433. Pflugers Arch. 2000. PMID: 11211107 - Stoichiometry of expressed KCNQ2/KCNQ3 potassium channels and subunit composition of native ganglionic M channels deduced from block by tetraethylammonium.
Hadley JK, Passmore GM, Tatulian L, Al-Qatari M, Ye F, Wickenden AD, Brown DA. Hadley JK, et al. J Neurosci. 2003 Jun 15;23(12):5012-9. doi: 10.1523/JNEUROSCI.23-12-05012.2003. J Neurosci. 2003. PMID: 12832524 Free PMC article.
Cited by
- Activation of expressed KCNQ potassium currents and native neuronal M-type potassium currents by the anti-convulsant drug retigabine.
Tatulian L, Delmas P, Abogadie FC, Brown DA. Tatulian L, et al. J Neurosci. 2001 Aug 1;21(15):5535-45. doi: 10.1523/JNEUROSCI.21-15-05535.2001. J Neurosci. 2001. PMID: 11466425 Free PMC article. - Coupling of the nucleotide P2Y4 receptor to neuronal ion channels.
Filippov AK, Simon J, Barnard EA, Brown DA. Filippov AK, et al. Br J Pharmacol. 2003 Jan;138(2):400-6. doi: 10.1038/sj.bjp.0705043. Br J Pharmacol. 2003. PMID: 12540532 Free PMC article. - Inhibition of KCNQ1-4 potassium channels expressed in mammalian cells via M1 muscarinic acetylcholine receptors.
Selyanko AA, Hadley JK, Wood IC, Abogadie FC, Jentsch TJ, Brown DA. Selyanko AA, et al. J Physiol. 2000 Feb 1;522 Pt 3(Pt 3):349-55. doi: 10.1111/j.1469-7793.2000.t01-2-00349.x. J Physiol. 2000. PMID: 10713961 Free PMC article. - Phosphatidylinositol 4,5-bisphosphate interactions with the HERG K(+) channel.
Bian JS, McDonald TV. Bian JS, et al. Pflugers Arch. 2007 Oct;455(1):105-13. doi: 10.1007/s00424-007-0292-5. Epub 2007 Jul 11. Pflugers Arch. 2007. PMID: 17622552 Review. - Kv7 and Kv11 channels in myometrial regulation.
Greenwood IA, Tribe RM. Greenwood IA, et al. Exp Physiol. 2014 Mar;99(3):503-9. doi: 10.1113/expphysiol.2013.075754. Epub 2013 Oct 11. Exp Physiol. 2014. PMID: 24121285 Free PMC article. Review.
References
- Abogadie FC, Vallis Y, Buckley NJ, Caulfield MP. Use of antisense-generating plasmids to probe the function of signal transduction proteins in primary neurons. In: Challiss RAJ, editor. Methods in molecular biology, Vol 83: Receptor signal transduction protocols. Humana; Totowa, NJ: 1997. pp. 217–225. - PubMed
- Bianchi L, Wible B, Arcangeli A, Taglialatela M, Morra F, Castaldo P, Crociani O, Rosati B, Faravelli L, Olivotto M, Wanke E. Herg encodes a K+ current highly conserved in tumours of different histogenesis: a selective advantage for cancer cells? Cancer Res. 1998;58:815–822. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases