Dynamic regulation of cpg15 during activity-dependent synaptic development in the mammalian visual system - PubMed (original) (raw)
Dynamic regulation of cpg15 during activity-dependent synaptic development in the mammalian visual system
R A Corriveau et al. J Neurosci. 1999.
Abstract
During visual system development, neural activity regulates structural changes in connectivity including axonal branching and dendritic growth. Here we have examined a role for the candidate plasticity gene 15 (cpg15), which encodes an activity-regulated molecule that can promote dendritic growth, in this process. We report that cpg15 is expressed in the cat visual system at relatively high levels in the lateral geniculate nucleus (LGN) but at very low levels in its synaptic target, layer 4 of the visual cortex. Prenatally, when cpg15 mRNA in the LGN is most abundant, expression is insensitive to action potential blockade by tetrodotoxin. Postnatally, activity regulation of cpg15 emerges in the LGN coincident with development of ocular dominance columns in the visual cortex. cpg15 can be detected in layers 2/3 and 5/6 of visual cortex postnatally, and expression in layers 2/3 is activity-regulated during known periods of activity-dependent plasticity for these layers. Localization and regulation of cpg15 expression in the visual system are consistent with a presynaptic role for CPG15 in shaping dendritic arbors of target neurons during activity-dependent synaptic rearrangements, both in development and adulthood.
Figures
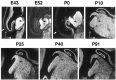
Fig. 1.
Regulation of feline _cpg15_mRNA expression in the LGN during normal development. In situ hybridization (using an 35S-labeled antisense riboprobe) was performed on brain sections at the level of the LGN (black arrows) from animals of the indicated ages. Results are presented in dark field. Sections were cut either in the horizontal [E43, E52, P0; anterior (A) and medial (M) are indicated] or coronal [P10, P25, P40, P91; dorsal (D) and M are indicated] plane. The white arrow in E43 and P0 is the visual cortex. Scale bar, 1 mm.
Fig. 2.
Regulation of feline cpg15 mRNA expression in the primary visual cortex during normal development.In situ hybridization was performed as described in Figure 1. A, Horizontal sections of visual cortex, with_arrowheads_ delimiting the cortical plate. Results are presented in dark field. The boxed area_indicates the approximate region shown in B. Anterior (A) and medial (M) are indicated. B, High magnification of feline_cpg15 in situ hybridization signal at P91 (dark field, left) and the image of an adjacent cresyl violet–stained section (bright field, right). Note that the vast majority of signal is found in layers 2/3, 5, and 6. The pial surface is up. wm, White matter. Scale bars: A, 1 mm; B, 0.5 mm.
Fig. 3.
Feline cpg15 mRNA levels decrease in the LGN (filled diamonds) and increase in primary visual cortex (filled and_open_ circles) during development. Error bars represent the SEM for 7–11 determinations (slides) obtained in two independent experiments, except for the E43 and P0 values, which are based on analysis of 4 and 3 determinations, respectively. Because results obtained for layers 2/3 and 5/6, quantified separately, were essentially identical, the numbers presented are only for layers 2/3 (filled circles).
Fig. 4.
Prenatal and early postnatal analyses of feline_cpg15_ regulation by endogenous neural activity in the LGN. A, In situ hybridization demonstrates little if any change in feline cpg15 mRNA levels in the prenatal LGN after 10 d (E42–E52) of intracranial application of the voltage-gated sodium channel blocker TTX. _Black arrows_indicate LGNs. B, Top, After 5 d of monocular TTX applied to the right eye between P6 and P11 (data shown for right LGN), the feline cpg15 signal remains unchanged in the eye-specific LGN layer corresponding to the blocked eye (layer A1; compare with control layer A).Middle, On an adjacent section BDNF mRNA levels decreased in layer A1, demonstrating that a successful activity blockade had been achieved. Bottom, Moreover, in an animal treated in parallel with vehicle only, BDNF mRNA levels remain unchanged in layer A1 (injection in right eye; right LGN shown). Note that at this time in development BDNF mRNA levels are low in the LGN. Scale bars, 1 mm.
Fig. 5.
Regulation of feline cpg15 mRNA levels in the LGN by visual experience. Monocular activity blockade was maintained for the indicated times by injection of TTX into the posterior chamber of the right eye. Dark-field photomicrographs of selected in situ hybridization results. An_asterisk_ indicates the eye-specific LGN layer receiving synaptic input from the treated eye (layer A on the left, contralateral to the blocked eye, and layer A1 on the_right_, ipsilateral to the blocked eye). Controls (LGNs ipsilateral to the injected eye; treatment was from P38 to P48) show a dramatic decrease in BDNF mRNA in layer A1, confirming the effectiveness of the activity blockade; no decrease is observed in feline cpg15 mRNA levels when vehicle alone is injected. All sections are in the coronal plane. Medial is toward the center of the figure (except for the BDNF control, in which medial is left), and dorsal is_up_. Scale bar, 1 mm.
Fig. 6.
Quantitation of feline cpg15 mRNA regulation in the LGN after monocular blockade. Data are presented for the left LGN, i.e., contralateral to the blocked eye. An index of activity regulation of feline cpg15 mRNA expression was obtained by dividing signal obtained in the eye-specific layer corresponding to the treated eye (A) by that obtained in the control layer (A1) (see Materials and Methods). Each column represents the mean ± SEM for three to five determinations from two to three independent animals.Asterisks indicate feline cpg15 ratios that are significantly different from controls shown on the_left_ (p < 0.01). The P38–P48 BDNF A/A1 ratio (number symbol) is significantly different from all other ratios (p < 0.001). The results indicate that feline cpg15 mRNA levels in the LGN are specifically regulated by visual experience after P20 and into adulthood. Analysis of data for the LGN ipsilateral to the injected eye (data not shown, but see Fig. 5) resulted in identical conclusions.
Fig. 7.
Activity regulation of feline cpg15_in primary visual cortex. Top, After monocular activity blockade for the indicated times, horizontal sections through the visual cortex, optimal for viewing ODCs, were prepared and analyzed by_in situ hybridization. Results are presented in dark-field optics. Note the periodicity of signal intensity, with several regions of decreased signal indicated by_arrowheads_ on each panel.A, Anterior. M, medial. Scale bar, 1 mm.Bottom, Signal intensity was quantified in the superficial layers (described in Materials and Methods) and found to have a spatial frequency of ∼1 mm, as would be expected if the periodicity observed corresponds to inputs from the control (higher signal) and activity-blocked (lower signal) eyes and therefore ocular dominance columns. Note that no such regular periodicity in signal was observed when the analysis was performed on visual cortex from an animal monocularly treated with vehicle from P61 to P64 (D).
Similar articles
- Extended plasticity of visual cortex in dark-reared animals may result from prolonged expression of cpg15-like genes.
Lee WC, Nedivi E. Lee WC, et al. J Neurosci. 2002 Mar 1;22(5):1807-15. doi: 10.1523/JNEUROSCI.22-05-01807.2002. J Neurosci. 2002. PMID: 11880509 Free PMC article. - Rapid regulation of brain-derived neurotrophic factor mRNA within eye-specific circuits during ocular dominance column formation.
Lein ES, Shatz CJ. Lein ES, et al. J Neurosci. 2000 Feb 15;20(4):1470-83. doi: 10.1523/JNEUROSCI.20-04-01470.2000. J Neurosci. 2000. PMID: 10662837 Free PMC article. - Brain-derived neurotrophic factor enhances expression of superior cervical ganglia clone 10 in lateral geniculate nucleus and visual cortex of developing kittens.
Imamura K, Morii H, Nakadate K, Yamada T, Mataga N, Watanabe Y, Mori N. Imamura K, et al. Eur J Neurosci. 2006 Feb;23(3):637-48. doi: 10.1111/j.1460-9568.2006.04592.x. Eur J Neurosci. 2006. PMID: 16487145 - Competitive interactions between retinal ganglion cells during prenatal development.
Shatz CJ. Shatz CJ. J Neurobiol. 1990 Jan;21(1):197-211. doi: 10.1002/neu.480210113. J Neurobiol. 1990. PMID: 2181063 Review. - The influence of action potentials on the development of the central visual pathway in mammals.
Kalil RE. Kalil RE. J Exp Biol. 1990 Oct;153:261-76. doi: 10.1242/jeb.153.1.261. J Exp Biol. 1990. PMID: 2280224 Review.
Cited by
- Thalamus-derived molecules promote survival and dendritic growth of developing cortical neurons.
Sato H, Fukutani Y, Yamamoto Y, Tatara E, Takemoto M, Shimamura K, Yamamoto N. Sato H, et al. J Neurosci. 2012 Oct 31;32(44):15388-402. doi: 10.1523/JNEUROSCI.0293-12.2012. J Neurosci. 2012. PMID: 23115177 Free PMC article. - Soluble cpg15 from Astrocytes Ameliorates Neurite Outgrowth Recovery of Hippocampal Neurons after Mouse Cerebral Ischemia.
Zhao JJ, Hu JX, Lu DX, Ji CX, Qi Y, Liu XY, Sun FY, Huang F, Xu P, Chen XH. Zhao JJ, et al. J Neurosci. 2017 Feb 8;37(6):1628-1647. doi: 10.1523/JNEUROSCI.1611-16.2016. Epub 2017 Jan 9. J Neurosci. 2017. PMID: 28069924 Free PMC article. - Neurobiology and cellular pathogenesis of glycolipid storage diseases.
Walkley SU. Walkley SU. Philos Trans R Soc Lond B Biol Sci. 2003 May 29;358(1433):893-904. doi: 10.1098/rstb.2003.1276. Philos Trans R Soc Lond B Biol Sci. 2003. PMID: 12803923 Free PMC article. Review. - Experience-dependent plasticity of mouse visual cortex in the absence of the neuronal activity-dependent marker egr1/zif268.
Mataga N, Fujishima S, Condie BG, Hensch TK. Mataga N, et al. J Neurosci. 2001 Dec 15;21(24):9724-32. doi: 10.1523/JNEUROSCI.21-24-09724.2001. J Neurosci. 2001. PMID: 11739581 Free PMC article. - AMPA receptors and their minions: auxiliary proteins in AMPA receptor trafficking.
Bissen D, Foss F, Acker-Palmer A. Bissen D, et al. Cell Mol Life Sci. 2019 Jun;76(11):2133-2169. doi: 10.1007/s00018-019-03068-7. Epub 2019 Apr 1. Cell Mol Life Sci. 2019. PMID: 30937469 Free PMC article. Review.
References
- Bodnarenko SR, Chalupa LM. Stratification of ON and OFF ganglion cell dendrites depends on glutamate-mediated afferent activity in the developing retina. Nature. 1993;364:144–146. - PubMed
- Campbell G, Ramoa AS, Stryker MP, Shatz CJ. Dendritic development of retinal ganglion cells after prenatal intracranial infusion of tetrodotoxin. Vis Neurosci. 1997;14:779–788. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY011894/EY/NEI NIH HHS/United States
- R37 EY002858/EY/NEI NIH HHS/United States
- EY11894/EY/NEI NIH HHS/United States
- R01 EY002858/EY/NEI NIH HHS/United States
- R29 EY011894/EY/NEI NIH HHS/United States
- EY02858/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous