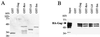Human immunodeficiency virus type 1 Gag polyprotein multimerization requires the nucleocapsid domain and RNA and is promoted by the capsid-dimer interface and the basic region of matrix protein - PubMed (original) (raw)
Human immunodeficiency virus type 1 Gag polyprotein multimerization requires the nucleocapsid domain and RNA and is promoted by the capsid-dimer interface and the basic region of matrix protein
M T Burniston et al. J Virol. 1999 Oct.
Abstract
The human immunodeficiency virus type 1 (HIV-1) Gag polyprotein directs the formation of virions from productively infected cells. Many gag mutations disrupt virion assembly, but little is known about the biochemical effects of many of these mutations. Protein-protein interactions among Gag monomers are believed to be necessary for virion assembly, and data suggest that RNA may modify protein-protein interactions or even serve as a bridge linking Gag polyprotein monomers. To evaluate the primary sequence requirements for HIV-1 Gag homomeric interactions, a panel of HIV-1 Gag deletion mutants was expressed in bacteria and evaluated for the ability to associate with full-length Gag in vitro. The nucleocapsid protein, the major RNA-binding domain of Gag, exhibited activity comparable to that of the complete polyprotein. In the absence of the nucleocapsid protein, relatively weak activity was observed that was dependent upon both the capsid-dimer interface and basic residues within the matrix domain. The relevance of the in vitro findings was confirmed with an assay in which nonmyristylated mutant Gags were assessed for the ability to be incorporated into virions produced by wild-type Gag expressed in trans. Evidence of the importance of RNA for Gag-Gag interaction was provided by the demonstration that RNase impairs the Gag-Gag interaction and that HIV-1 Gag interacts efficiently with Gags encoded by distantly related retroviruses and with structurally unrelated RNA-binding proteins. These results are consistent with models in which Gag multimerization involves indirect contacts via an RNA bridge as well as direct protein-protein interactions.
Figures
FIG. 1
In vitro assay for HIV-1 Gag polyprotein multimerization. (A) Schematic diagram of the three proteins that were expressed in bacteria. (B and C) The results of a typical binding experiment in which GST or GST-Gag was purified from bacterial lysates by incubation with glutathione-agarose beads. Unbound proteins were removed by washing. The beads with bound proteins were then incubated with bacterial lysate containing HA-Gag. Beads were washed three more times, and proteins that remained bound were eluted by boiling in SDS and then were analyzed by SDS-PAGE. Proteins that remained associated with the glutathione beads were visualized by staining with Coomassie blue (B) or by Western blotting with anti-HA antibody (C). The input lane shows 10% of the HA-Gag lysate added to the binding reaction. The positions of migration of GST, GST-Gag, and HA-Gag are indicated with arrows.
FIG. 2
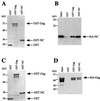
Interaction of HIV-1 HA-Gag amino-terminal deletion mutants with the full-length HIV-1 Gag polyprotein. (A) The primary structure of the HIV-1 Gag polyprotein is depicted schematically at the top. Numbers refer to the position of encoding nucleotides with respect to the 5′ end of the 5′ LTR. Vertical bars indicate the location of viral protease recognition sites. The major proteolytic products are labeled using standard nomenclature: MA indicates matrix protein, CA indicates capsid protein, and NC indicates nucleocapsid protein. MHR, major homology region. Shaded horizontal bars indicate the residues expressed by the mutants, and horizontal lines indicate residues that were deleted. Gag-binding activity of each mutant, quantitated as described in the text, is presented at the right of the figure: +++ indicates activity 50 to 100% of wild type, ++ indicates activity 20 to 50% of wild type, and − indicates activity less than 5% of wild type. (B to F) Immunoblots showing the HIV-1 Gag polyprotein-binding activity of select amino-terminal deletion mutants. Primary data for the remaining mutants is not shown due to lack of space. GST or GST-Gag was purified from bacterial lysates by incubation with glutathione-agarose beads. Beads with associated proteins were washed and then incubated with lysate from bacteria expressing the indicated HA-Gag mutant proteins: 5′Δ1184 (B), 5′Δ1320 (C), 5′Δ1632 (D), 5′Δ1920 (E), or 5′Δ1962 (F). After extensive washing, bound proteins were subjected to SDS-PAGE and were visualized by Western blotting with anti-HA antibody. In each case the input lane shows 10% of the HA-mutant fusion protein lysate added to the binding reaction.
FIG. 3
Interaction of HIV-1 Gag polyprotein carboxyl-terminal deletion mutants with full-length Gag polyprotein. (A) Schematic diagram as in Fig. 2 depicting the primary structure of the carboxyl-terminal mutants. Gag polyprotein-binding activity is indicated at the right of the figure: +++ indicates activity 50 to 100% of wild type, + indicates activity 5 to 10% of wild type, − indicates activity less than 5% of wild type; U.E. indicates that expression of the mutant protein was unstable. (B to F) Immunoblots presented as in Fig. 2 showing the HIV-1 Gag polyprotein-binding activity of select carboxyl-terminal deletion mutants 3′Δ2093 (B), 3′Δ2007 (C), 3′Δ1906 (D), 3′Δ1878 (E), and 3′Δ1715 (F). After extensive washing, bound proteins were subjected to SDS-PAGE and were visualized by Western blotting with anti-HA antibody. In each case, the input lane shows 10% of the HA-mutant fusion protein lysate added to the binding reaction.
FIG. 4
Carboxyl-terminal CA residues contribute to Gag polyprotein multimerization. (A) Schematic diagram showing the location and hydrophobic residues constituting the CA-dimer interface (23). Amino acids W184 and M185 are shown in bold. In vitro binding experiments were performed as described for Fig. 1 with wild-type HA-Gag (B) or with HA-Gag possessing the W184A (C) or M185A (D) mutation. The inputs of the three HA-Gag proteins were normalized to each other by serial dilution (data not shown). The amino acid numbering is with respect to the amino-terminal residue of CA.
FIG. 5
Basic residues in matrix contribute to the multimerization of Gag mutants in which NC is deleted. (A) Schematic diagram as in Fig. 2 depicting the primary structure of the mutants tested here. The 11 amino acid residues deleted from mutant 5′Δ850-884 are shown in blow-up at the top left of the schematic, and basic residues are indicated with bold letters. Gag-binding activity of each mutant is presented at the right of the figure: +++ indicates activity 50 to 100% of wild type, ++ indicates activity 20 to 50% of wild type, − indicates activity less than 5% of wild type. (B to F) Immunoblots presented as in Fig. 2 showing the HIV-1 Gag polyprotein-binding activity of mutants 5′Δ1320/3′Δ2093 (B), 5′Δ1320/3′Δ2007 (C), 5′Δ850-884 (D), 5′Δ850-884/3′Δ2093 (E), and 5′Δ850-884/3′Δ2007 (F). In each case the input lane shows 10% of the HA-mutant fusion protein lysate added to the binding reaction.
FIG. 6
NC associates with the Gag polyprotein. GST, GST-Gag, or GST-NC was purified from bacterial lysates by incubation with glutathione-agarose beads. Beads with associated proteins were washed and then incubated with lysate from bacteria expressing HA-NC (A and B) or HA-Gag (C and D). After extensive washing, bound proteins were subjected to SDS-PAGE and were visualized with Coomassie blue (A and C) or by Western blotting with anti-HA antibody (B and D). Input lanes show 10% of the HA-fusion protein lysate added to the binding reaction. The positions of the migrations of GST, GST-Gag, GST-NC, HA-NC, and HA-Gag are indicated by arrows.
FIG. 7
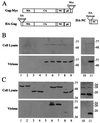
In vivo assay for HIV-1 Gag-Gag interaction. The Gag fusion proteins expressed in this assay are shown schematically in panel A. Gag-Myc is an HIV-1 Gag polyprotein with a Myc-epitope tag fused at the carboxyl terminus. HA-Gag, either wild type or bearing one of the mutations listed below, and HA-NC have an HA-epitope tag fused at the amino terminus so that neither protein is myristylated when expressed in eukaryotic cells. 293T cells were transfected with plasmids expressing wild-type HA-Gag (lane 1) or HA-Gag-W184A (lane 2), HA-Gag-3′Δ1878 (lane 3), HA-Gag-3′Δ1878/W184A (lane 4), or HA-NC (lane 10) mutants. The Gag-Myc expression plasmid was transfected alone (lane 5) or in combination with expression plasmids for wild-type HA-Gag (lane 6) or HA-Gag-W184A (lane 7), HA-Gag-3′Δ1878 (lane 8), HA-Gag-3′Δ1878/W184A (lane 9), or HA-NC (lane 11) mutants. Cell lysates (upper panels in B and C) and purified virions (lower panels in B and C) were processed for Western blotting and probed with either primary, anti-Myc antibody (B) or primary, anti-HA antibody (C). Positions of migration of molecular mass markers in kilodaltons are indicated on the right.
FIG. 8
RNase A inhibits HIV-1 Gag polyprotein multimerization. Bacterial lysate containing HA-Gag was incubated for 1 h at 37°C with (+) or without (−) RNase A. (A) Ethidium-stained agarose gel of the HA-Gag-containing bacterial lysates. M indicates the marker lane. The positions of migration of bacterial chromosomal DNA (chDNA) and bacterial RNA (RNA) are shown. GST or GST-Gag was purified from bacterial lysates by incubation with glutathione-agarose beads. Unbound proteins were removed by washing. Beads with bound proteins were incubated for 1 h at 37°C with (+) or without (−) RNase A. Beads were washed three times and then used in binding experiments with the bacterial lysates shown in panel A as described in Fig. 1. Beads were washed three more times, and proteins that remained bound were eluted by boiling in SDS and then analyzed by SDS-PAGE. GST-Gag that remained associated with the glutathione beads was visualized by staining with Coomassie blue (B). The stability of HA-Gag to RNase treatment is monitored in panel C, which shows a Western blot with anti-HA antibody of the protein that failed to associate with GST-Gag bound to glutathione-agarose beads. (D) Western blot with anti-HA antibody showing the HA-Gag protein that remained associated with the glutathione-agarose beads at the end of the binding reaction. The input lane shows 10% of the RNase-treated HA-Gag fusion protein lysate added to the binding reaction.
FIG. 9
HIV-1 Gag interacts in vitro with Gag polyproteins encoded by other retroviruses. In vitro binding experiments were performed as described for Fig. 1. (A and B) The ability of wild-type HIV-1 HA-Gag protein to bind to GST–HIV-1 Gag was compared with its ability to bind to GST fusions with the Gag polyproteins encoded by SIVMAC239, FIVPETALUMA, visna virus, RSV, or HFV, as indicated above the lanes. Proteins that remained associated with the glutathione beads were visualized by staining with Coomassie blue (A) or by Western blotting with anti-HA antibody (B). The gels in panels C and D, Coomassie blue stained and Western blot, respectively, show a similar comparison between GST fusion proteins with HIV-1 NC and GST fusion protein with HFV NC (62). The input lane shows 10% of the HA-Gag lysate added to the binding reaction. The position of migration of HA-Gag is indicated.
FIG. 10
HIV-1 Gag interacts in vitro with heterologous RNA-binding proteins. In vitro binding experiments were performed as described for Fig. 1. The ability of wild-type HIV-1 HA-Gag protein to bind to GST–HIV-1 Gag was compared with its ability to bind to GST fusions with HIV-1 Rev (Rev), human ribosomal protein L8 (L8), or human autoantigen small nuclear ribonucleoprotein Sm-D (Sm), as indicated above the lanes. Proteins that remained associated with the glutathione beads were visualized by staining with Coomassie blue (A) or by Western blotting with anti-HA antibody (B). The input lane shows 10% of the HA-Gag lysate added to the binding reaction. The position of migration of HA-Gag is indicated.
Similar articles
- The role of nucleocapsid of HIV-1 in virus assembly.
Dawson L, Yu XF. Dawson L, et al. Virology. 1998 Nov 10;251(1):141-57. doi: 10.1006/viro.1998.9374. Virology. 1998. PMID: 9813210 - Translation elongation factor 1-alpha interacts specifically with the human immunodeficiency virus type 1 Gag polyprotein.
Cimarelli A, Luban J. Cimarelli A, et al. J Virol. 1999 Jul;73(7):5388-401. doi: 10.1128/JVI.73.7.5388-5401.1999. J Virol. 1999. PMID: 10364286 Free PMC article. - Proteolytic processing of the p2/nucleocapsid cleavage site is critical for human immunodeficiency virus type 1 RNA dimer maturation.
Shehu-Xhilaga M, Kraeusslich HG, Pettit S, Swanstrom R, Lee JY, Marshall JA, Crowe SM, Mak J. Shehu-Xhilaga M, et al. J Virol. 2001 Oct;75(19):9156-64. doi: 10.1128/JVI.75.19.9156-9164.2001. J Virol. 2001. PMID: 11533179 Free PMC article. - How HIV-1 Gag Manipulates Its Host Cell Proteins: A Focus on Interactors of the Nucleocapsid Domain.
Klingler J, Anton H, Réal E, Zeiger M, Moog C, Mély Y, Boutant E. Klingler J, et al. Viruses. 2020 Aug 13;12(8):888. doi: 10.3390/v12080888. Viruses. 2020. PMID: 32823718 Free PMC article. Review. - HIV-1 gag proteins: diverse functions in the virus life cycle.
Freed EO. Freed EO. Virology. 1998 Nov 10;251(1):1-15. doi: 10.1006/viro.1998.9398. Virology. 1998. PMID: 9813197 Review.
Cited by
- Molecular Determinants Directing HIV-1 Gag Assembly to Virus-Containing Compartments in Primary Macrophages.
Inlora J, Chukkapalli V, Bedi S, Ono A. Inlora J, et al. J Virol. 2016 Sep 12;90(19):8509-19. doi: 10.1128/JVI.01004-16. Print 2016 Oct 1. J Virol. 2016. PMID: 27440886 Free PMC article. - HIV-1 Gag associates with specific uropod-directed microdomains in a manner dependent on its MA highly basic region.
Llewellyn GN, Grover JR, Olety B, Ono A. Llewellyn GN, et al. J Virol. 2013 Jun;87(11):6441-54. doi: 10.1128/JVI.00040-13. Epub 2013 Mar 27. J Virol. 2013. PMID: 23536680 Free PMC article. - The carboxy-terminal fragment of nucleolin interacts with the nucleocapsid domain of retroviral gag proteins and inhibits virion assembly.
Bacharach E, Gonsky J, Alin K, Orlova M, Goff SP. Bacharach E, et al. J Virol. 2000 Dec;74(23):11027-39. doi: 10.1128/jvi.74.23.11027-11039.2000. J Virol. 2000. PMID: 11069998 Free PMC article. - Intracellular targeting of Gag proteins of the Drosophila telomeric retrotransposons.
Rashkova S, Athanasiadis A, Pardue ML. Rashkova S, et al. J Virol. 2003 Jun;77(11):6376-84. doi: 10.1128/jvi.77.11.6376-6384.2003. J Virol. 2003. PMID: 12743295 Free PMC article. - Mobility of human immunodeficiency virus type 1 Pr55Gag in living cells.
Gomez CY, Hope TJ. Gomez CY, et al. J Virol. 2006 Sep;80(17):8796-806. doi: 10.1128/JVI.02159-05. J Virol. 2006. PMID: 16912326 Free PMC article.
References
- Barklis E, McDermott J, Wilkens S, Fuller S, Thompson D. Organization of HIV-1 capsid proteins on a lipid monolayer. J Biol Chem. 1998;273:7177–7180. - PubMed
- Berkowitz R, Fisher J, Goff S P. RNA packaging. Curr Top Microbiol Immunol. 1996;214:177–218. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources