Inhibition of protein phosphatase 2A induces serine/threonine phosphorylation, subcellular redistribution, and functional inhibition of STAT3 - PubMed (original) (raw)
. 1999 Sep 14;96(19):10620-5.
doi: 10.1073/pnas.96.19.10620.
M Nielsen, S T Christensen, J Brockdorff, K Kaltoft, A M Engel, S Skov, C Brender, C Geisler, A Svejgaard, J Rygaard, V Leick, N Odum
Affiliations
- PMID: 10485875
- PMCID: PMC17932
- DOI: 10.1073/pnas.96.19.10620
Inhibition of protein phosphatase 2A induces serine/threonine phosphorylation, subcellular redistribution, and functional inhibition of STAT3
A Woetmann et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Signal transducers and activators of transcription (STATs) are rapidly phosphorylated on tyrosine residues in response to cytokine and growth factor stimulation of cell surface receptors. STATs hereafter are translocated to the nucleus where they act as transcription factors. Recent reports suggest that serine phosphorylation of STATs also is involved in the regulation of STAT-mediated gene transcription. Here, we studied the role of serine/threonine phosphatases in STAT3 signaling in human antigen-specific CD4(+) T cell lines and cutaneous T cell lymphoma lines, expressing a constitutively activated STAT3. We show that an inhibitor of protein phosphatases (PPs) PP1/PP2A, calyculin A, induces (i) phosphorylation of STAT3 on serine and threonine residues, (ii) inhibition of STAT3 tyrosine phosphorylation and DNA binding activity, and (iii) relocation of STAT3 from the nucleus to the cytoplasm. Similar results were obtained with other PP2A inhibitors (okadaic acid, endothall thioanhydride) but not with inhibitors of PP1 (tautomycin) or PP2B (cyclosporine A). Pretreatment with the broad serine/threonine kinase inhibitor staurosporine partly blocked the calyculin A-induced STAT3 phosphorylation, whereas inhibitors of serine/threonine kinases, such as mitogen-activated protein kinase-1 extracellular-regulated kinase-kinase, mitogen-activated protein p38 kinase, and phosphatidylinositol 3-kinase, did not. In conclusion, we provide evidence that PP2A plays a crucial role in the regulation of STAT3 phosphorylation and subcellular distribution in T cells. Moreover, our findings suggest that the level of STAT3 phosphorylation is balanced between a staurosporine-sensitive kinase(s) and PP2A.
Figures
Figure 1
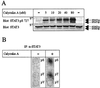
(A) CA induces serine phosphoryaltion of STAT3 in vivo. Resting, antigen-specific human CD4+ T cells were incubated with increasing concentrations of CA (5–80 nM) for 60 min, lysed in lysis buffer, applied to SDS/PAGE as described in Materials and Methods, and immunoblotted with anti-STAT3 pS727 (Upper), stripped and reblotted with anti-STAT3 (Lower). (B) Amino acid analysis of 32P-orthophosphate-labeled STAT3. 32P-labeled proteins of interest, representing STAT3 proteins from cutaneous T lymphoma cells incubated with or without CA for 60 min, were cut out from the poly(vinylidene difluoride) membrane, hydrolyzed in 6 M HCl, and separated by thin-layer electrophoresis. The 32P-labeled phosphoamino acids were detected by autoradiography as described in Materials and Methods.
Figure 2
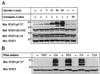
(A) Inhibition of PP1/PP2A induces rapid (<1 hr) serine phosphorylation of STAT3. T lymphoma cells were incubated with CA (80 nM) or CyA (400 ng/ml) for 5–60 min, lysed, applied to SDS/PAGE, and immunoblotted with anti-STAT3 pS 727 pAb, stripped and reblotted with anti-STAT3 (S21320), anti-STAT3 (K-15), and anti-STAT5. (B) PP2A-specific, but not a PP1-specific, inhibitor, induced STAT3 serine phosphorylation. T lymphoma cells were incubated in either TAU (500 nM), OA (500 nM), ETA (100 μM), CA (80 nM), or CyA (400 ng/ml) for 60 min, lysed in lysis buffer and applied to SDS-PAGE, and immunoblotted with anti-STAT3 pS727 (Upper) and stripped, and reblotted with anti-STAT3 (Lower).
Figure 3
(A) PD98059 inhibits CA-induced activation of ERK p42/44, but not CA-induced STAT3 serine phosphorylation. T lymphoma cells were preincubated with or without a MEK inhibitor: PD98059 at 50 μM for 60 min, before incubation with CA (80 nM), OA (500 nM), or CyA (400 ng/ml) for 60 min, lysed and applied to SDS/PAGE, and immunoblotted with anti-phospho-p42/44 ERK, stripped and reblotted with anti-p42/44 ERK, anti-STAT3 pS727, or anti-STAT3. (B) Inhibition of staurosporine-sensitive kinases inhibits CA-induced serine phosphorylation of STAT3. In parallel experiments antigen-specific human CD4+ T cells were preincubated in either medium H7 at 200 μM, MEK inhibitor PD98059 at 50 μM (PD), or staurosporine (STA) at 100 nM for 60 min before incubation in medium, the PP1/PP2A-specific inhibitor CA at 80 nM or the PP2B-specific inhibitor CyA at 400 ng/ml for an additional 60 min. Cells were lysed and applied to SDS/PAGE as described in Materials and Methods, and immunoblotted with anti-STAT3 pS727 (Upper) and stripped and reblotted with anti-STAT3 (Lower).
Figure 4
CA inhibits the function of STAT3. (A) CA-induced serine phosphorylation of STAT3 precedes decrease in phospho-tyrosine STAT3. T lymphoma cells were incubated with CA (80 nM) or CyA (400 ng/ml) for 1–60 min, lysed, applied to SDS/PAGE, and immunoblotted with anti-STAT3 pY705 (Top), stripped and reblotted with anti-STAT3 pS727 (Middle), or anti-STAT3 (Bottom). (B) Inhibition of PP2A by CA inhibits DNA binding of STAT3 from T lymphoma cells. Cytoplasmic extracts from T lymphoma cells incubated with or without CA (80 nM) for 60 min were analyzed by affinity purification of STAT proteins by using biotinylated DNA probes [IL-2Rα (GASd or GASp)], c-fos (hSIE), or ICAM-1 (pIRE)]. STAT/DNA complexes were analyzed by Western blotting with anti-STAT3 mAb.
Figure 5
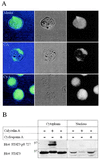
Inhibition of PP2A modulates the subcellular distribution of STAT3. (A) Confocal laser scanning microscopy analysis of STAT3 location in T lymphoma cells incubated with solvent (media, Top), 80 nM CA (Middle), or 400 ng/ml CyA (Bottom). Cells were fixed in 1% paraformaldehyde in PBS and stained with anti-STAT3 (#9132) followed by confocal laser scanning microscopy analysis as described in Materials and Methods. (Right) Green fluorescence. (Center) Blue interference contrast. (Left) combination of both. (B) Serine phosphorylation inhibits nuclear translocation of STAT3. Cytoplasmic and nuclear extracts from T lymphoma cells incubated in medium CA (80 nM) or CyA (400 ng/ml) for 60 min were prepared as described in Materials and Methods and subsequently immunoblotted with anti-STAT3 pS727 (Upper) and stripped and reblotted with anti-STAT3 (Lower).
Similar articles
- Role of protein phosphatase 2A in mGluR5-regulated MEK/ERK phosphorylation in neurons.
Mao L, Yang L, Arora A, Choe ES, Zhang G, Liu Z, Fibuch EE, Wang JQ. Mao L, et al. J Biol Chem. 2005 Apr 1;280(13):12602-10. doi: 10.1074/jbc.M411709200. Epub 2005 Jan 20. J Biol Chem. 2005. PMID: 15661743 - Protein phosphatase 2A plays a critical role in interleukin-2-induced beta 2-integrin dependent homotypic adhesion in human CD4+ T cell lines.
Brockdorff J, Nielsen M, Svejgaard A, Dobson P, Röpke C, Geisler C, Odum N. Brockdorff J, et al. Cytokine. 1997 May;9(5):333-9. doi: 10.1006/cyto.1996.0173. Cytokine. 1997. PMID: 9195132 - Regulation of angiotensin II-induced phosphorylation of STAT3 in vascular smooth muscle cells.
Liang H, Venema VJ, Wang X, Ju H, Venema RC, Marrero MB. Liang H, et al. J Biol Chem. 1999 Jul 9;274(28):19846-51. doi: 10.1074/jbc.274.28.19846. J Biol Chem. 1999. PMID: 10391929 - Cross-talk between angiotensin II and interleukin-6-induced signaling through Stat3 transcription factor.
Bhat GJ, Baker KM. Bhat GJ, et al. Basic Res Cardiol. 1998;93 Suppl 3:26-9. doi: 10.1007/s003950050203. Basic Res Cardiol. 1998. PMID: 9879441 Review. - Serine/threonine phosphorylation in cytokine signal transduction.
McCubrey JA, May WS, Duronio V, Mufson A. McCubrey JA, et al. Leukemia. 2000 Jan;14(1):9-21. doi: 10.1038/sj.leu.2401657. Leukemia. 2000. PMID: 10637471 Review.
Cited by
- Signal transducer and activator of transcription (STAT) signalling and T-cell lymphomas.
Mitchell TJ, John S. Mitchell TJ, et al. Immunology. 2005 Mar;114(3):301-12. doi: 10.1111/j.1365-2567.2005.02091.x. Immunology. 2005. PMID: 15720432 Free PMC article. Review. - Avicin D: a protein reactive plant isoprenoid dephosphorylates Stat 3 by regulating both kinase and phosphatase activities.
Haridas V, Nishimura G, Xu ZX, Connolly F, Hanausek M, Walaszek Z, Zoltaszek R, Gutterman JU. Haridas V, et al. PLoS One. 2009;4(5):e5578. doi: 10.1371/journal.pone.0005578. Epub 2009 May 18. PLoS One. 2009. PMID: 19440292 Free PMC article. - Protein phosphatase 2A controls the activity of histone deacetylase 7 during T cell apoptosis and angiogenesis.
Martin M, Potente M, Janssens V, Vertommen D, Twizere JC, Rider MH, Goris J, Dimmeler S, Kettmann R, Dequiedt F. Martin M, et al. Proc Natl Acad Sci U S A. 2008 Mar 25;105(12):4727-32. doi: 10.1073/pnas.0708455105. Epub 2008 Mar 13. Proc Natl Acad Sci U S A. 2008. PMID: 18339811 Free PMC article. - Structural determinants of mitochondrial STAT3 targeting and function.
Marié IJ, Lahiri T, Önder Ö, Elenitoba-Johnson KSJ, Levy DE. Marié IJ, et al. Mitochondrial Commun. 2024;2:1-13. doi: 10.1016/j.mitoco.2024.01.001. Epub 2024 Jan 10. Mitochondrial Commun. 2024. PMID: 38500969 Free PMC article. - 14-3-3ζ interacts with stat3 and regulates its constitutive activation in multiple myeloma cells.
Zhang J, Chen F, Li W, Xiong Q, Yang M, Zheng P, Li C, Pei J, Ge F. Zhang J, et al. PLoS One. 2012;7(1):e29554. doi: 10.1371/journal.pone.0029554. Epub 2012 Jan 18. PLoS One. 2012. PMID: 22279540 Free PMC article.
References
- Leaman D W, Leung S, Li X, Stark G R. FASEB J. 1996;10:1578–1588. - PubMed
- Leonard W J, O’Shea J J. Annu Rev Immunol. 1998;16:293–322. - PubMed
- Liu K D, Gaffen S L, Goldsmith M A. Curr Immunol. 1998;10:271–278. - PubMed
- Mufson R A. FASEB J. 1997;11:37–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous