Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains - PubMed (original) (raw)
Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains
G C Kopen et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Stem cells are a valuable resource for treating disease, but limited access to stem cells from tissues such as brain restricts their utility. Here, we injected marrow stromal cells (MSCs) into the lateral ventricle of neonatal mice and asked whether these multipotential mesenchymal progenitors from bone marrow can adopt neural cell fates when exposed to the brain microenvironment. By 12 days postinjection, MSCs migrated throughout the forebrain and cerebellum without disruption to the host brain architecture. Some MSCs within the striatum and the molecular layer of the hippocampus expressed glial fibrillary acidic protein and, therefore, differentiated into mature astrocytes. MSCs also populated neuron rich regions including the Islands of Calleja, the olfactory bulb, and the internal granular layer of the cerebellum. A large number of MSCs also were found within the external granular layer of the cerebellum. In addition, neurofilament positive donor cells were found within the reticular formation of the brain stem, suggesting that MSCs also may have differentiated into neurons. Therefore, MSCs are capable of producing differentiated progeny of a different dermal origin after implantation into neonatal mouse brains. These results suggest that MSCs are potentially useful as vectors for treating a variety of central nervous system disorders.
Figures
Figure 1
Murine MSC cultures before and after immunodepletion. Seven-day-old murine MSC cultures before (A and C) and after (B and D) immunodepletion were stained with Geimsa (A and B) or with a rat anti-mouse CD11b antibody, detected with an FITC-conjugated anti-rat secondary, and counterstained with 4′,6-diamidino-2-phenylindole (C and D).
Figure 2
Differentiation of immunodepleted MSCs into chondrocytes and adipocytes in vitro. (A) Photomicrograph of immunodepleted MSCs cultured in micromass for 6 weeks and stained with toluidine blue. (B) Higher magnification image of A reveals rich glycosaminoglycan deposition pericellular to hypertrophic chondrocytes embedded within lacunae. (C) Phase contrast image of immunodepleted MSCs cultured for 1 week in adipogenic medium. (D) Photomicrograph of cells in C stained with Oil Red O and counterstained with toluidine blue. (A and D, ×400; B, ×1,000; C, ×100.)
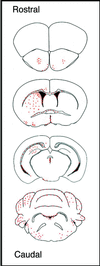
Figure 3
Distribution of immunodepleted MSCs throughout brain at 12 days postinjection. Red dots indicate the regions of BrdUrd-labeled MSCs in coronal sections. Similar results were obtained with three mice.
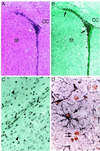
Figure 4
Immunohistochemical localization of BrdUrd-labeled MSCs in forebrain. Hematoxylin/eosin (A)- or anti-BrdUrd (B)-stained serial sections of striatum and lateral ventricle, ipsilateral to the injection site at bregma. (C) High power magnification of BrdUrd-labeled cells in the external capsule. Photomicrograph is from same section as B but shows a more lateral field. (D) MSC-derived astrocyte in the molecular layer of the hippocampus double labeled with anti-BrdUrd and anti-GFAP (black). Arrows, BrdUrd-labeled nuclei; arrow-heads, nuclei negative for BrdUrd-labeling. (A and B, ×40; C, ×400; D, ×1,000.)
Figure 5
Localization of BrdUrd-labeled MSCs in neural rich regions of forebrain. (A) Low magnification of forebrain showing BrdUrd-labeled cells in the Islands of Calleja that are rich in granule neurons. (B) Higher magnification of boxed region in A. Arrows indicate typical positive cells; arrowheads indicate typical negative cells. (C) Hematoxylin/eosin-stained section of the subependyma of the olfactory bulb. (D) Adjacent section stained for BrdUrd. (A, C, and D, ×40; B, ×400.)
Figure 6
Localization of BrdUrd-labeled MSCs in cerebellum. Hematoxylin/eosin (A) or anti-BrdUrd (B) staining of serial sections reveal MSCs within the EGL, molecular layer (ML), and IGL of the cerebellum. Red arrowheads indicate negative staining of Purkinje cells. (C) MSCs in the reticular formation of the brain stem triple-labeled with anti-BrdUrd, anti-GFAP, and anti-neurofilament. (×400.) (Inset) Higher magnification reveals neurofilament staining (red reaction product) in the cytoplasmic processes of numerous BrdUrd (yellow reaction product)-labeled MSCs. (×1,000.)
Similar articles
- A comparison of neural differentiation and retinal transplantation with bone marrow-derived cells and retinal progenitor cells.
Tomita M, Mori T, Maruyama K, Zahir T, Ward M, Umezawa A, Young MJ. Tomita M, et al. Stem Cells. 2006 Oct;24(10):2270-8. doi: 10.1634/stemcells.2005-0507. Stem Cells. 2006. PMID: 17008430 - Multilineage potential of stable human mesenchymal stem cell line derived from fetal marrow.
Nagai A, Kim WK, Lee HJ, Jeong HS, Kim KS, Hong SH, Park IH, Kim SU. Nagai A, et al. PLoS One. 2007 Dec 5;2(12):e1272. doi: 10.1371/journal.pone.0001272. PLoS One. 2007. PMID: 18060066 Free PMC article. - Mesenchymal stem cells spontaneously express neural proteins in culture and are neurogenic after transplantation.
Deng J, Petersen BE, Steindler DA, Jorgensen ML, Laywell ED. Deng J, et al. Stem Cells. 2006 Apr;24(4):1054-64. doi: 10.1634/stemcells.2005-0370. Epub 2005 Dec 1. Stem Cells. 2006. PMID: 16322639 - From bone to brain: human skeletal stem cell therapy for stroke.
Zhou S. Zhou S. Cent Nerv Syst Agents Med Chem. 2011 Jun 1;11(2):157-63. doi: 10.2174/187152411796011376. Cent Nerv Syst Agents Med Chem. 2011. PMID: 21521166 Review. - Central and peripheral nerve regeneration by transplantation of Schwann cells and transdifferentiated bone marrow stromal cells.
Dezawa M. Dezawa M. Anat Sci Int. 2002 Mar;77(1):12-25. doi: 10.1046/j.0022-7722.2002.00012.x. Anat Sci Int. 2002. PMID: 12418080 Review.
Cited by
- Neuronal differentiation of adipose-derived stem cells and their transplantation for cerebral ischemia.
Tian G, Zhou J, Wang J, Xu B, Li L, Zhu F, Han J, Li J, Zhang S, Luo X. Tian G, et al. Neural Regen Res. 2012 Sep 5;7(25):1992-9. doi: 10.3969/j.issn.1673-5374.2012.25.011. Neural Regen Res. 2012. PMID: 25624830 Free PMC article. - The tyrosine kinase inhibitor dasatinib induces a marked adipogenic differentiation of human multipotent mesenchymal stromal cells.
Borriello A, Caldarelli I, Basile MA, Bencivenga D, Tramontano A, Perrotta S, Della Ragione F, Oliva A. Borriello A, et al. PLoS One. 2011;6(12):e28555. doi: 10.1371/journal.pone.0028555. Epub 2011 Dec 2. PLoS One. 2011. PMID: 22164306 Free PMC article. - Mesenchymal stem cell-based treatments for stroke, neural trauma, and heat stroke.
Hsuan YC, Lin CH, Chang CP, Lin MT. Hsuan YC, et al. Brain Behav. 2016 Aug 3;6(10):e00526. doi: 10.1002/brb3.526. eCollection 2016 Oct. Brain Behav. 2016. PMID: 27781140 Free PMC article. Review. - In vivo animal stroke models: a rationale for rodent and non-human primate models.
Tajiri N, Dailey T, Metcalf C, Mosley YI, Lau T, Staples M, van Loveren H, Kim SU, Yamashima T, Yasuhara T, Date I, Kaneko Y, Borlongan CV. Tajiri N, et al. Transl Stroke Res. 2013 Jun;4(3):308-21. doi: 10.1007/s12975-012-0241-2. Transl Stroke Res. 2013. PMID: 23682299 Free PMC article. Review. - Neuronal transcription program induced in hippocampal cells cocultured with bone marrow derived mesenchymal cells.
Majeed S, Aziz A, Simjee SU. Majeed S, et al. Heliyon. 2020 Oct 8;6(10):e05083. doi: 10.1016/j.heliyon.2020.e05083. eCollection 2020 Oct. Heliyon. 2020. PMID: 33083598 Free PMC article.
References
- Evans M J, Kaufman M H. Nature (London) 1981;292:154–156. - PubMed
- Pedersen R A. Reprod Fertil Dev. 1994;6:543–552. - PubMed
- Morrison S J, Uchida N, Weissman I L. Annu Rev Cell Dev Biol. 1994;11:35–71. - PubMed
- Hall P A, Watt F M. Development (Cambridge, UK) 1989;106:619–633. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources